Application of 3beta-hydroxynorerythrosuamine-3-O-beta-D-glucopyranoside to preparing antitumor pharmaceutic preparation
A technology of glucopyranoside and anti-tumor drugs, which is applied in the field of natural medicines and chemical medicines to achieve good medicinal prospects, reduce the number of cell clones, and inhibit proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
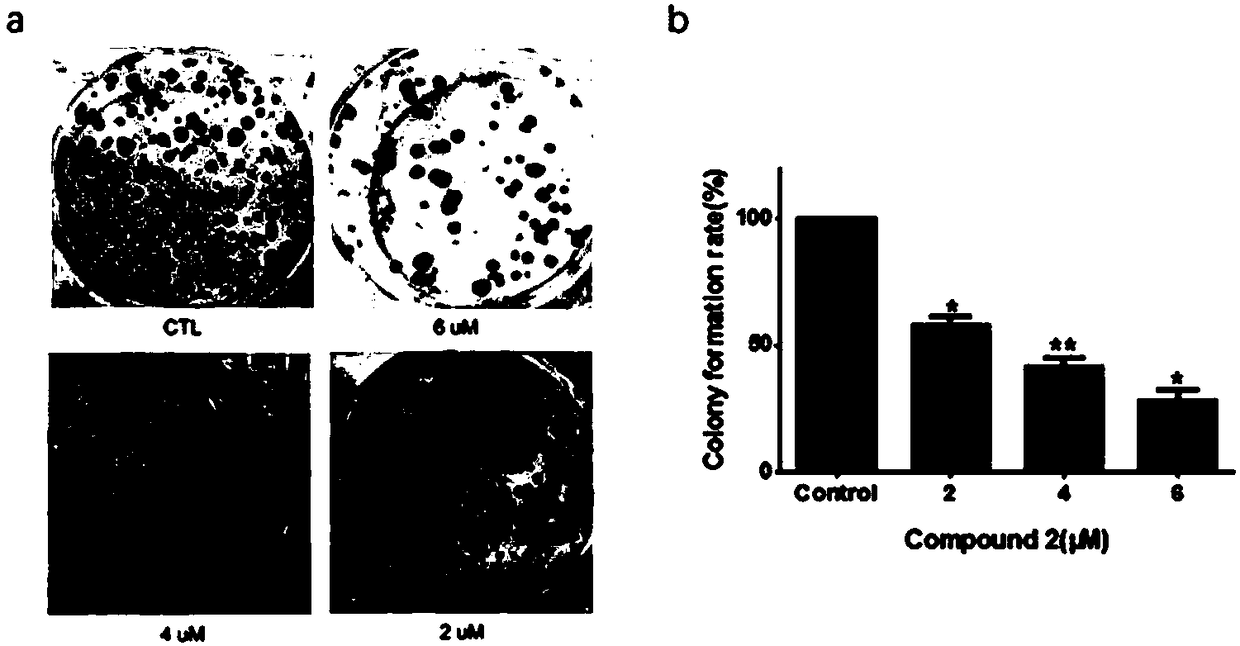
[0023] Inhibition of tumor cell proliferation by 3β-hydroxydesmethylglucothreamide 3-O-β-D-glucopyranoside.
[0024] Test method: cells grown in logarithmic phase (human breast cancer cell MCF-7, human breast cancer cell MDA-MB-231, human breast cancer cell MDA-MB-453, human non-small cell carcinoma cell A549, human non-small cell carcinoma cell Cell carcinoma cells NCI-H1299, human liver cancer cells HepG2, human liver cancer cells Bel-7402, human ovarian cancer cells A2780, human cervical cancer cells Hela, human colon cancer cells HCT-8, human gastric cancer cells BGC-823, human glial cells Tumor cells (U87) were digested, centrifuged, counted, and 1*10 6 cells / ml inoculated in 96-well culture plate, 100 μL per well, 37°C, 5% CO 2 After incubating in the incubator for 24 hours, add 3β-hydroxynormothreamide 3-O-β-D-glucopyranoside with a concentration gradient, and set solvent control and blank control at the same time. After 24 hours of incubation, add 20 μL to each well ...
Embodiment 2
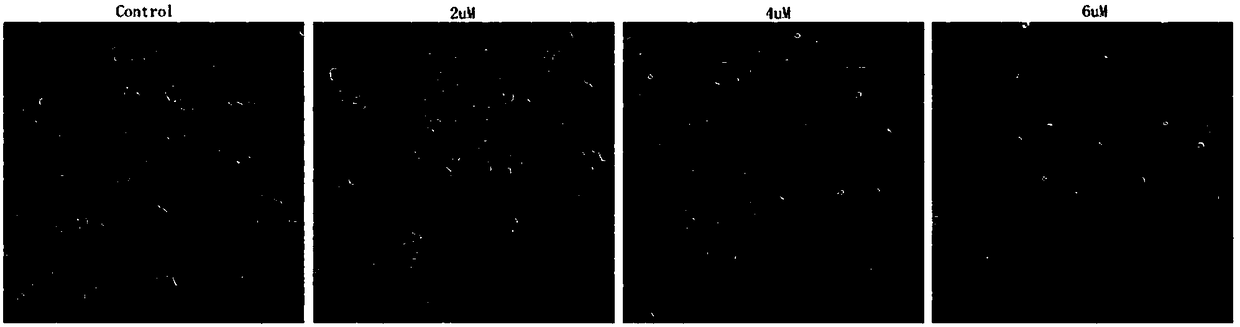
[0033] Morphological observation of human breast cancer MCF-7 cells acted on by 3β-hydroxydesmethylglucothreamide 3-O-β-D-glucopyranoside.
[0034] Test method: cells MCF-7 (human breast cancer cells) in the logarithmic growth phase were digested, centrifuged, counted, and 1*10 5 cells / ml inoculated in 6-well culture plate, 3ml per well, 37°C, 5% CO 2 After incubating in the incubator for 18 hours, 3β-hydroxynorgamthrein 3-O-β-D-glucopyranoside (0, 2, 4 and 6 μM) was added in a gradient concentration, incubated for 48 hours, and observed under a microscope .
[0035] Test results: if figure 1 As shown, the normal cells are plump and have a strong refractive index. After adding 2 μM of 3β-hydroxydesmegamthreamide 3-O-β-D-glucopyranoside, the cells become elongated, scattered, and a few parts are round. With the increase of drug concentration, the number of rounded cells increased and the refractive index decreased. At 6 μM, some cells had already floated in the culture mediu...
Embodiment 3
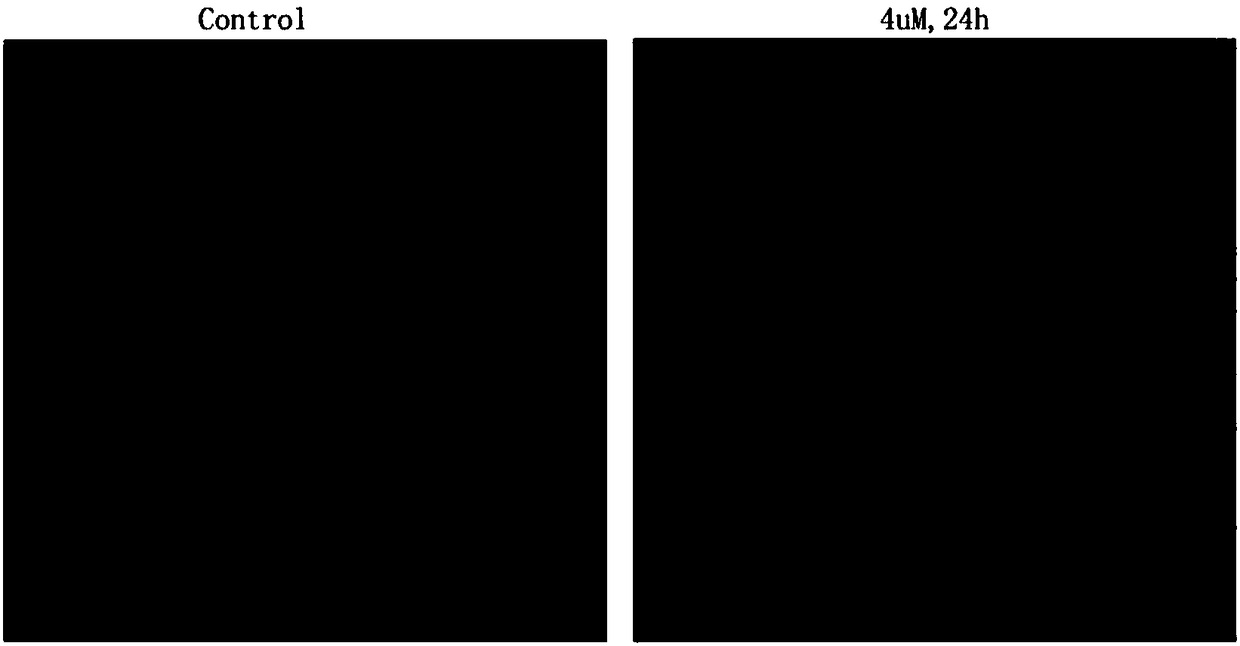
[0037] Hoechst staining of human breast cancer MCF-7 cells treated with 3β-hydroxynorgamthreamide 3-O-β-D-glucopyranoside.
[0038] Test method: cells MCF-7 (human breast cancer cells) in the logarithmic growth phase were digested, centrifuged, counted, and 1*10 5 cells / ml inoculated in 6-well culture plate, 3ml per well, 37°C, 5% CO 2 After incubating in the incubator for 18 hours, add 3β-hydroxynorgamthreamide 3-O-β-D-glucopyranoside with a concentration of 4 μM. After culturing for 24 hours, suck out the culture medium, wash once with PBS, and add Hoechst dye was incubated in an incubator. After 15 minutes, the dye was sucked off, washed again with PBS, and observed with a fluorescent inverted microscope.
[0039] Test results: Under a fluorescent inverted microscope, compared with normal cells that only showed weak fluorescence, the volume of MCF-7 cells after 3-O-β-D-glucopyranoside was treated for 24 hours, smaller, chromatin shrinkage, bright blue nuclei and obvious ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com