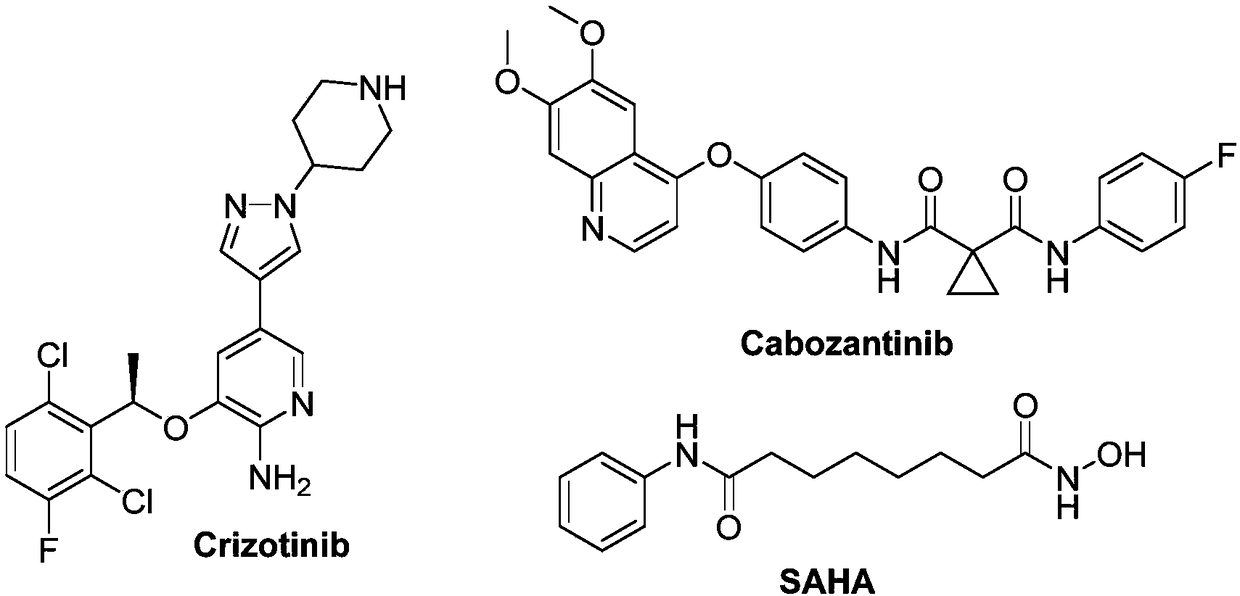
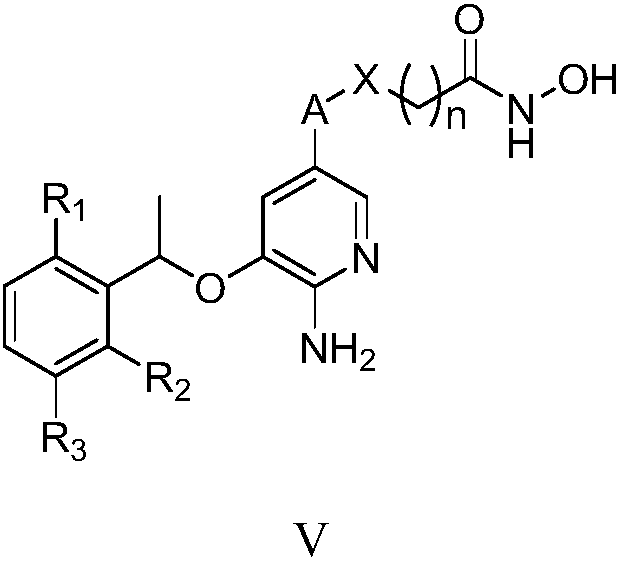
Aminopyridine derivative containing hydroxamic acid fragment as well as application thereof to anti-tumor aspect
An aminopyridine, hydroxamic acid technology, applied in antitumor drugs, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as poor curative effect and easy generation of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
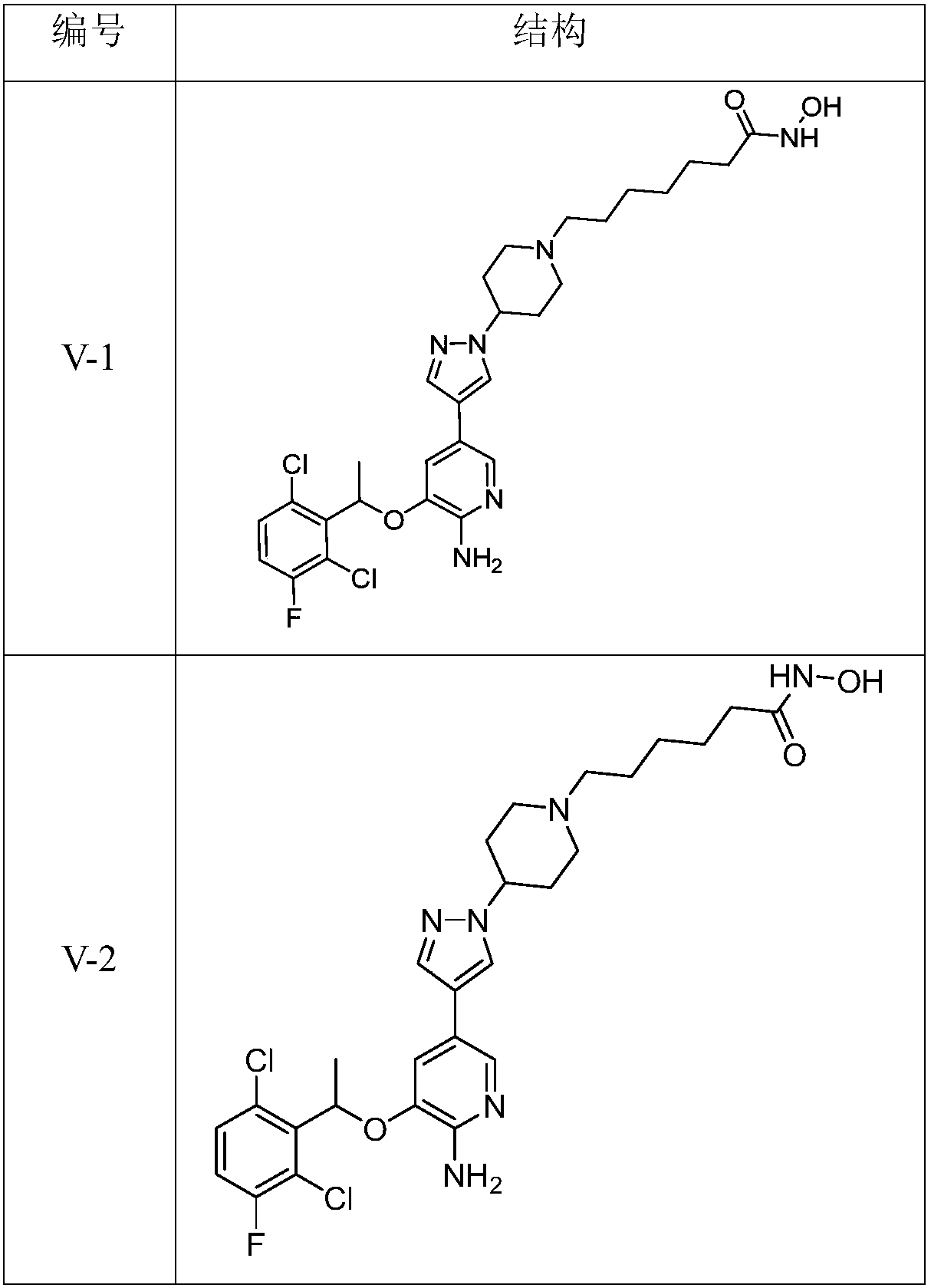
[0103] 7-(4-(4-(6amino-5-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin)3-yl)pyrazol-1-yl)piperidine Synthesis of -1-yl-N-hydroxyheptanamide (V-1) and its hydrochloride
[0104] 5-bromo-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-2-aminopyridine (0.5g, 1.3mmol), 1-(4-piperidine-1- (7-Ethyl heptanoate)-4-pyrazole pinacol boron ester (0.68g, 1.5mmol), KOH (0.58g, 10.4mmol), hydroxylamine hydrochloride (0.36g, 5.3mmol) as raw materials, according to class V Compound Synthesis General Method Scheme 1 to synthesize V-1 to obtain 0.35 g of the target compound with a yield of 45%.
[0105] ESI-MS[M+H] + :m / z 593.22
[0106] 1 H NMR (400MHz, DMSO-d 6 )δppm: δ7.78(s,1H),7.63(m,1H),7.52(s,1H),7.42(m,1H),7.20(m,1H),6.89(m,1H),6.14(q ,J=6.7Hz,1H),4.23(s,1H),3.15(m,3H),2.58(s,2H),2.45(s,2H),2.18(m,5H),1.84(m,2H) ,1.60(s,3H),1.34(s,3H),1.26(m,3H)
[0107] Compound V-1 (0.20g, 0.3mmol) was dissolved in isopropanol, concentrated hydrochloric acid was added dropwise, a white solid was pr...
Embodiment 2
[0109] 6-(4-(4-(6amino-5-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin)3-yl)pyrazol-1-yl)piperidine Synthesis of -1-yl-N-hydroxycaproamide (V-2)
[0110] 5-bromo-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-2-aminopyridine (0.5g, 1.3mmol), 1-(4-piperidine-1- (6-Ethyl hexanoate)-4-pyrazole pinacol boron ester (0.65g, 1.5mmol), KOH (0.58g, 10.4mmol), hydroxylamine hydrochloride (0.36g, 5.3mmol) as raw materials, according to Class V Compound Synthesis General Method Scheme 1 to synthesize V-2 to obtain 0.39 g of the target compound with a yield of 52%.
[0111] ESI-MS[M+H] + :m / z 579.21
[0112] 1 H NMR (400MHz, DMSO-d 6 )δppm: δ10.49(s,1H),8.81(s,1H),8.00(s,1H),7.75(s,1H),7.58(m,1H),7.53(s,1H),7.45(m ,1H),6.90(s,1H),6.09(q,J=6.7Hz,1H),5.67(s,2H),4.36(m,1H),4.02(m,2H),3.16(m,2H) ,2.97(s,2H),1.99(s,2H),1.80(m,4H),1.60(m,4H),1.23(s,3H).
Embodiment 3
[0114] 5-(4-(4-(6amino-5-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin)3-yl)pyrazol-1-yl)piperidine Synthesis of -1-yl-N-hydroxypentanamide (V-3)
[0115] 5-bromo-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-2-aminopyridine (0.5g, 1.3mmol), 1-(4-piperidine-1- (5-Ethyl pentanoate)-4-pyrazole pinacol boron ester (0.64g, 1.5mmol), KOH (0.58g, 10.4mmol), hydroxylamine hydrochloride (0.36g, 5.3mmol) as raw materials, according to Class V Compound Synthesis General Method Scheme 1 to synthesize V-3 to obtain 0.37 g of the target compound with a yield of 51%.
[0116] ESI-MS[M+H] + :m / z 565.20
[0117] 1 H NMR (400MHz, DMSO-d 6 )δppm: δ10.50(s,1H),8.81(s,1H),8.00(s,1H),7.75(s,1H),7.58(m,1H),7.53(s,1H),7.45(m ,1H),6.90(s,1H),6.09(q,J=6.7Hz,1H),5.67(s,2H),4.36(m,1H),4.02(m,4H),3.16(m,1H) ,2.97(s,2H),1.99(s,3H),1.80(m,4H),1.60(m,5H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com