Manufacturing method of controllable antibacterial tracheal stent
A technology of tracheal stent and manufacturing method, which is applied in the field of manufacture of controllable antibacterial tracheal stent, and can solve problems such as bacterial colonization and reproduction that are difficult to remove, lack of cellular immune function, and repeated airway infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
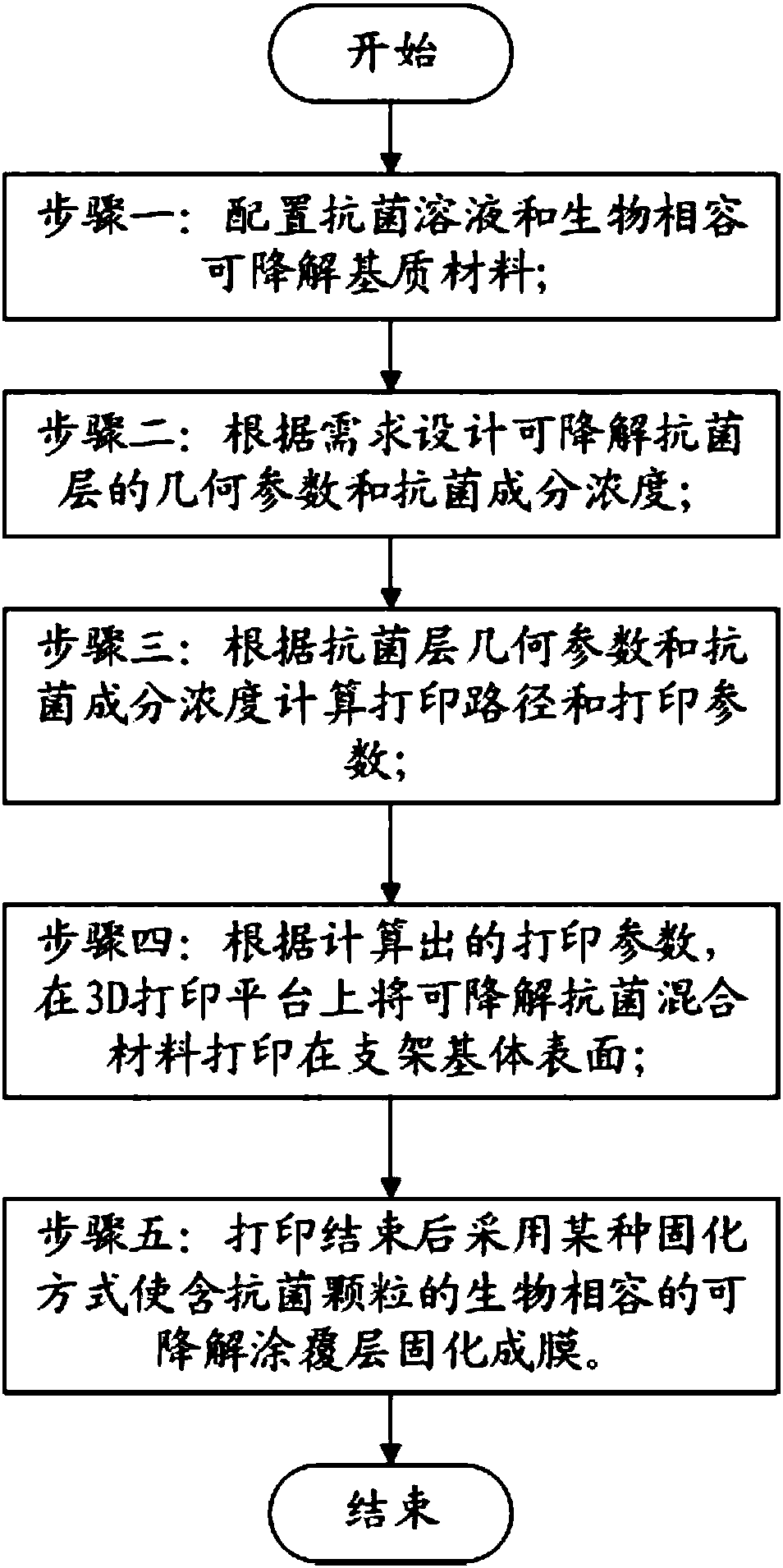
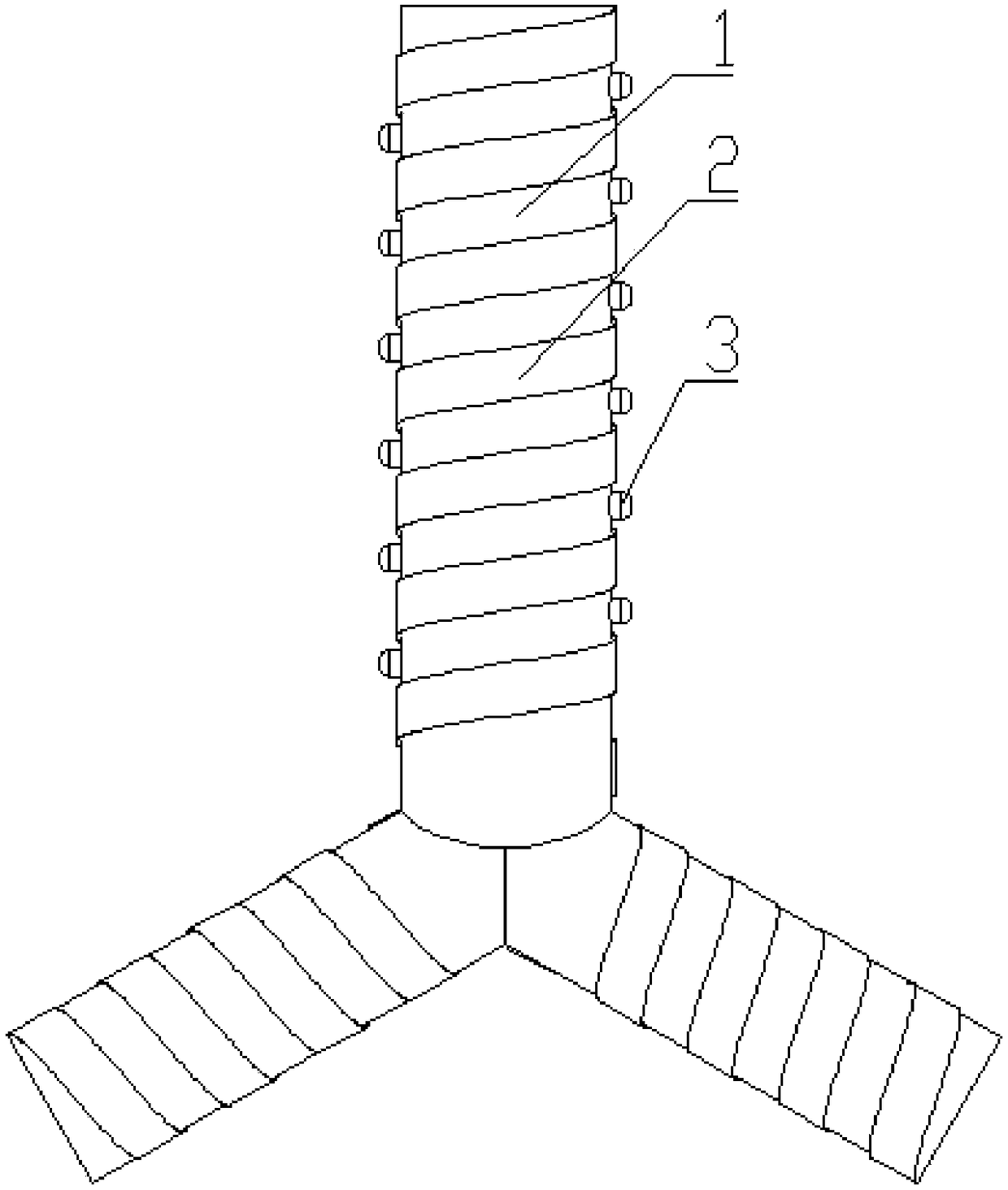
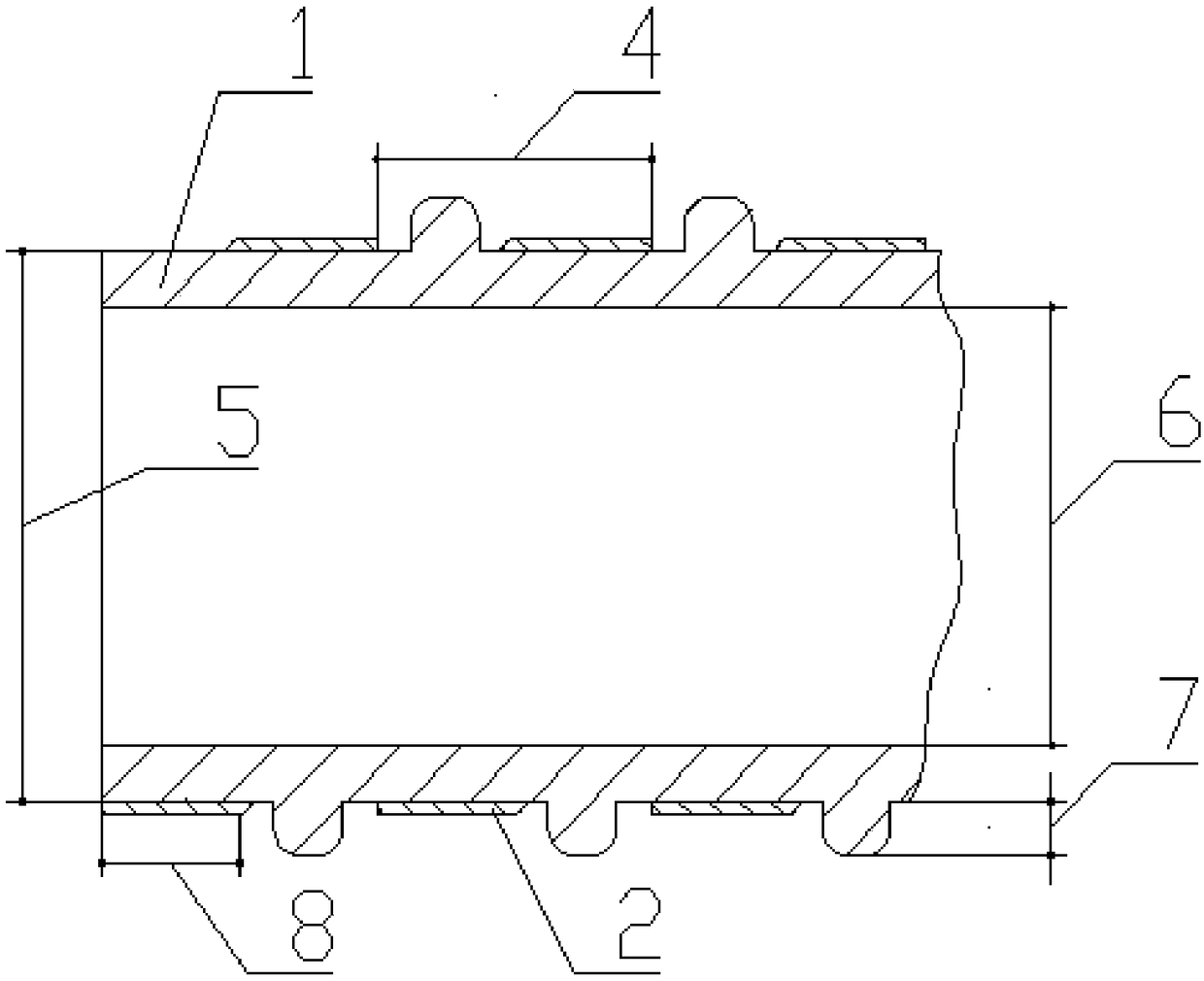
[0036] like figure 1 As shown, the controllable antibacterial tracheal stent manufacturing method of the present embodiment includes the following steps:
[0037] Step 1: configuring antibacterial solution (such as nano-silver solution) and biocompatible degradable matrix material (such as chitosan and gelatin mixed solution);
[0038] Step 2: Determine the geometric parameters and the concentration of antibacterial components of the degradable antibacterial layer according to the requirements;
[0039] Step 3: Calculate the printing path and printing parameters according to the geometric parameters of the antibacterial layer and the concentration of antibacterial components;
[0040] Step 4: Print the degradable antibacterial hybrid material on the surface of the stent matrix on the 3D printing platform according to the calculated printing parameters;
[0041] Step 5: After printing, a certain curing method is used to cure the biocompatible and degradable coating layer cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com