A method for an aseptic assembly of a multi-component medical device and a kit therefor
A medical device, multi-component technology, applied in the field of multi-component medical devices, can solve the problems of inactivation, difficulty, sensitive unit shielding, etc., and achieve the effect of cost reduction and sterilization volume reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
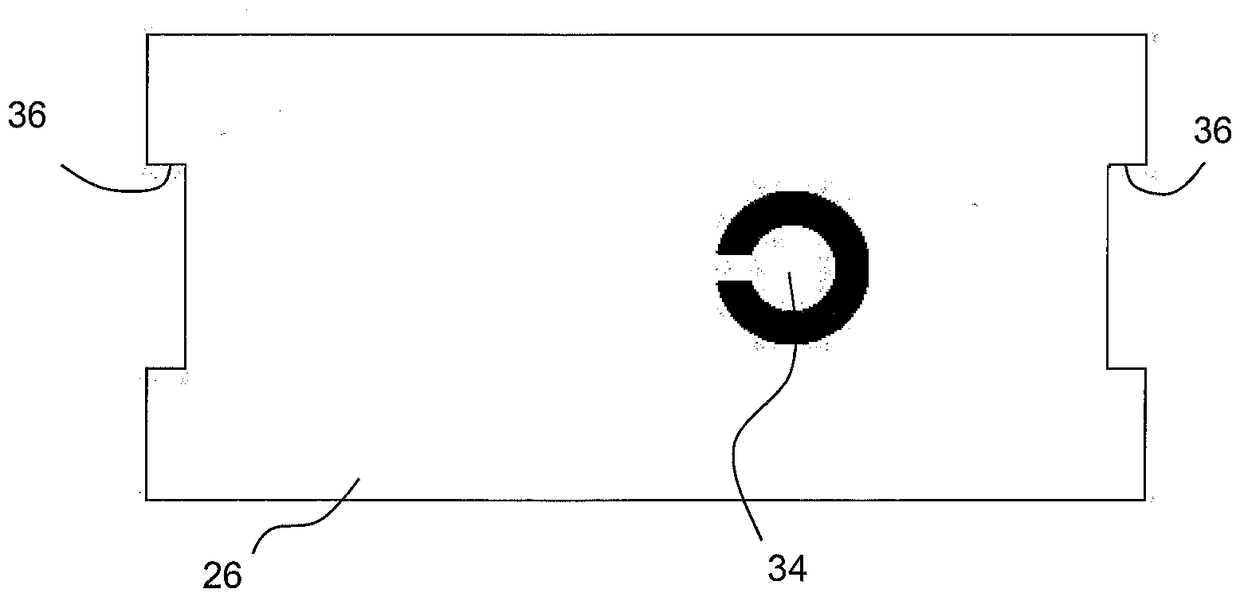
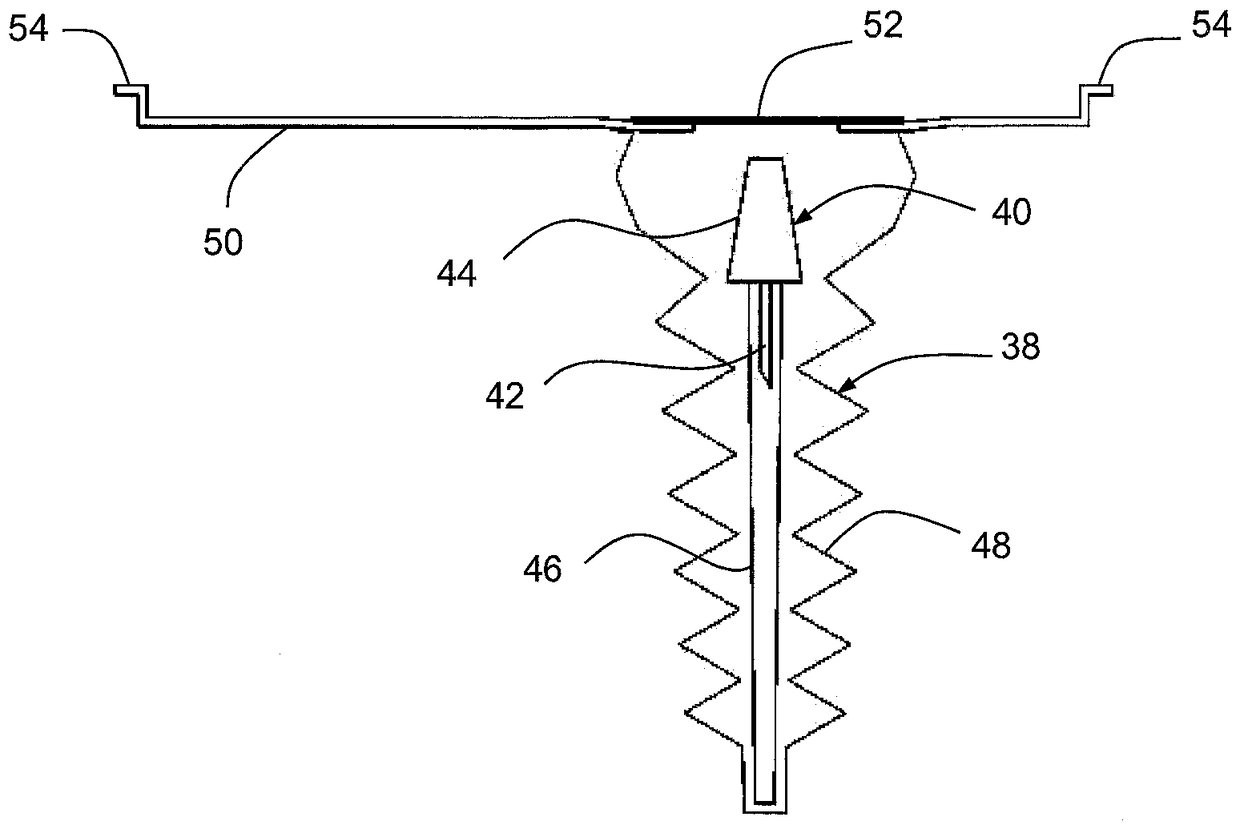
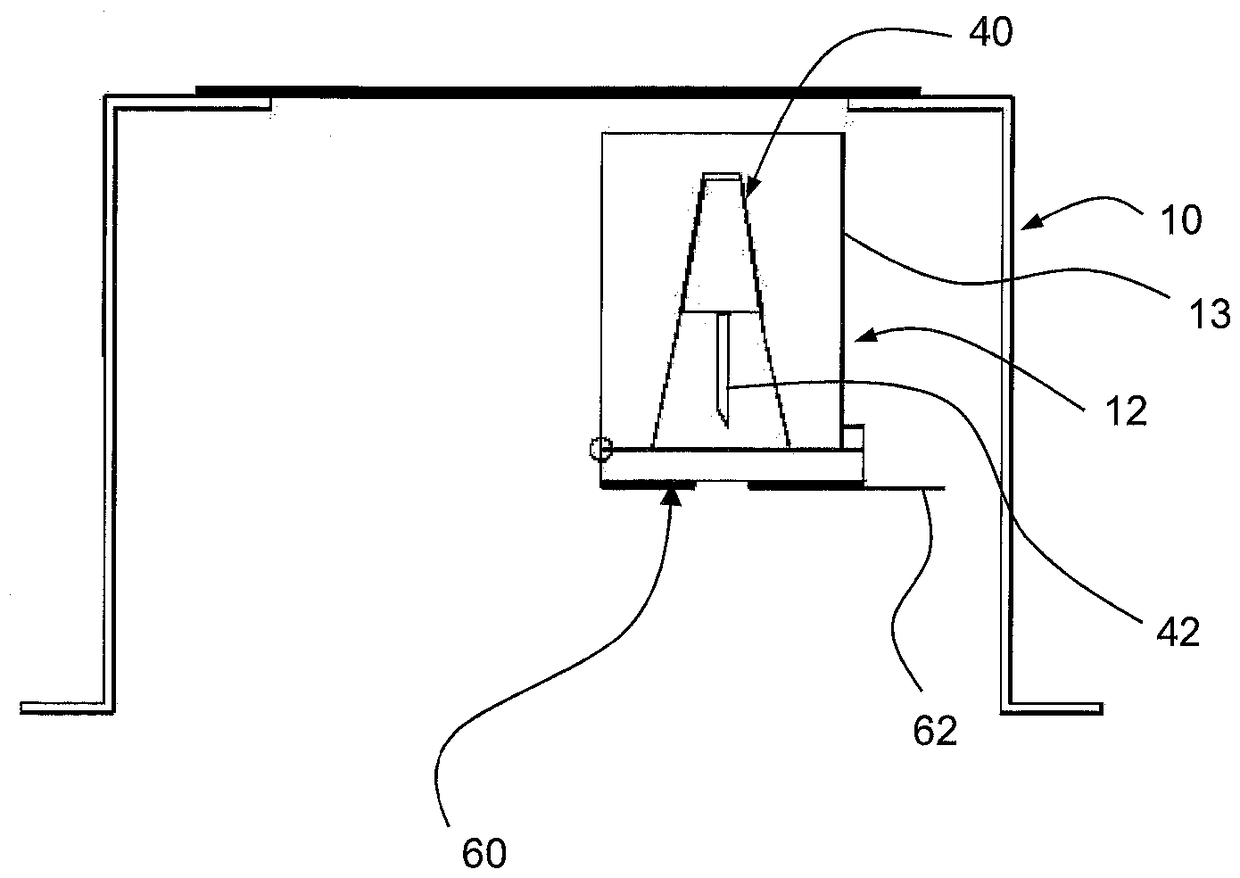
[0032] figure 1 Shown is a first container 10 comprising a first component 12 of a multi-component medical device. In this embodiment, the first component 12 comprises an applicator unit 13 combined with a patch 14 comprising an electronic component 16 and an adhesive pad 18 . The first container 10 has a rigid wall 20 , a transparent flexible wall 22 , a gas permeable membrane 24 and a cover 26 provided with a first rupturable portion 28 and coated with an adhesive layer 30 covered by a liner 32 .
[0033] as from figure 2 As evident in the figure, the first rupturable portion 28 is preferably formed from aluminum foil as a circular insert and reinforced by a central fin 34 which provides the cover 26 with a bendable connecting link 4 . Advantageously, the cover 26 is configured with a transverse cutout 36 as a positioning aid, as will be explained further below.
[0034] exist figure 1 In the state shown in , the first component 12 can be sterilized using gas sterilizat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com