A method for preparing cosmetic fragrance intermediates by selective epoxidation of nano functional materials
A nano-functional, iron oxide technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve separation difficulties, low selectivity, low yield, etc. problems, to achieve the effect of less dosage, optimized catalytic system, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
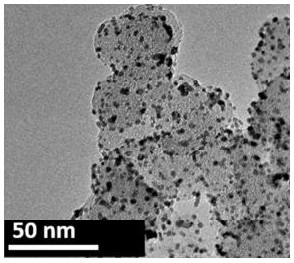
[0045] Prepare the nano-functional catalytic material as follows:
[0046] 1) Add 100ml of anhydrous tetrahydrofuran, 10mmol of manganese(II) acetylacetonate, 4.0g of iron oxide magnetic nanoparticles (average particle size 20nm), and 1ml of oleic acid into a 500ml three-necked flask and stir to dissolve to obtain the first dispersion;
[0047] 2) Add 13 g of cucurbit [7] urea hydrochloride hydrate to 100 ml of tetrahydrofuran / water mixed solution (volume ratio tetrahydrofuran / water = 2:1), reflux for 24 hours, and then lower the temperature to 50-60 ° C and ultrasonically disperse to obtain the second The dispersion is ready for use;
[0048] 3) Raise the temperature of the first dispersion to 50-60°C, and then use a peristaltic pump to drop the second dispersion into the first dispersion at a rate of 5ml / min. After the addition, keep stirring for 30-60 minutes to obtain the third Dispersions;
[0049] 4) Under ultrasonic conditions, add ammonia water dropwise to the third di...
Embodiment 2
[0053] Catalyst performance characterization:
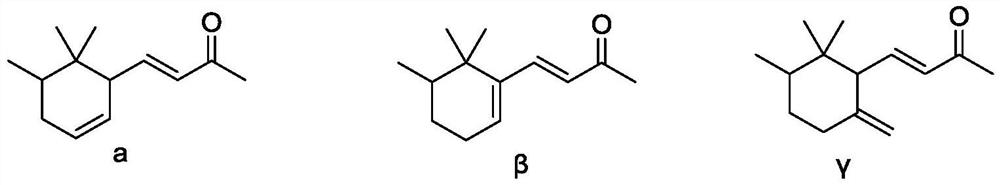
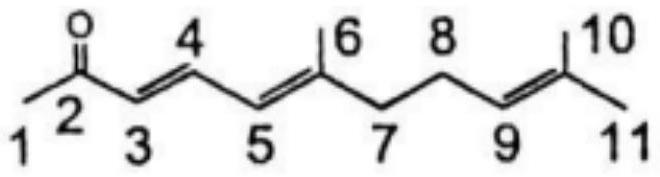
[0054] The catalyst prepared in Example 1 is used to catalyze pseudoionone, investigate the impact of different solvents on the reaction, and the reaction conditions are as follows:
[0055] 1) Dissolve the substrate pseudoionone (0.19g, ~1.0mmol) in 2ml of solvent in a parallel synthesizer, add nano-functional catalytic material (15mg, ~8.0%wt) and stir to disperse evenly;
[0056] 2) Lower the temperature of the system to 5°C, and then use a pipette gun to add dropwise a hydrogen peroxide solution (0.34g, ~3.0eq) with a concentration of 30%wt;
[0057] 3) After the dropwise addition, heat up to 40-50°C at a heating rate of 1-5°C / h and keep the temperature for reaction. Take the reaction solution every 2 hours to detect the concentration of the substrate pseudoionone by GC-MS. When there is another change, it is defaulted as the end of the reaction, and the conversion rate of different solvent substrates is counted. -Dien-2-ke...
Embodiment 3
[0063] Using acetone as a solvent to optimize the amount of catalyst and hydrogen peroxide, the steps are as follows:
[0064] 1) Dissolve the substrate pseudoionone (0.19g, ~1.0mmol) in 2ml of acetone in a parallel synthesizer, add nano-functional catalytic material (1-57mg, 0.5%wt~30.0%wt) and stir to disperse evenly;
[0065] 2) Lower the temperature of the system to 5°C, and then use a pipette gun to drop a hydrogen peroxide solution (0.12g-0.48g, 1.0-4.0eq) with a concentration of 30%wt;
[0066] 3) After the dropwise addition, heat up to 40-50°C at a heating rate of 1-5°C / h and keep the temperature for reaction. Take the reaction solution every 2 hours to detect the concentration of the substrate pseudoionone by GC-MS. When there is another change, it is defaulted as the end of the reaction, and the conversion rate of different solvent substrates is counted. -Dien-2-ketone is a standard substance, adopts the target product that GC positioning generates, and counts its c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com