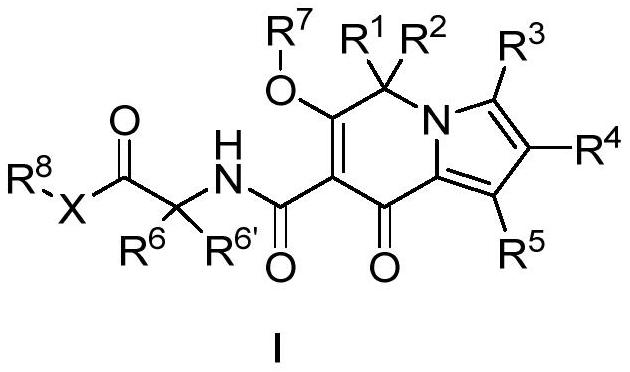
Indoxazine derivatives and their application in medicine
A kind of technology of indolazine, compound, be used in its preparation and its application in medicine, the field of indolizine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0190] (6'-Hydroxy-8'-oxo-8'H-spiro[cyclopropane-1,5'-indolezine]-7'-carbonyl)glycine ( 1 )
[0191]
[0192] first step
[0193] 1-(1H-pyrrol-1-yl)cyclopropane-1-carboxylic acid ( 1a )
[0194]
[0195] Add 1 ml of concentrated hydrochloric acid to a mixture of 2,5-dimethoxytetrahydrofuran (3.16 g, 24 mmol) and 20 ml of water and stir at room temperature for 1 hour; then add solid sodium acetate (3.28 g, 40 ml mol) and 1-aminocyclopropane-1-carboxylic acid (2.02 g, 20 mmol). The mixture was stirred overnight at room temperature; then diluted with water, neutralized to about pH 4 by adding 1M hydrochloric acid; then extracted with ethyl acetate; the ethyl acetate layer was separated, then washed twice with dilute aqueous sodium chloride; The solid was dried in ethyl acetate; the desiccant was removed by filtration, and the filtrate was spin-dried on a rotary evaporator; the residue was purified by column chromatography to obtain 1.8 g of product 1a . 1 H NMR (500MH...
Embodiment 2
[0210] (6'-Hydroxy-8'-oxo-8'H-spiro[cyclopropane-1,5'-indolezine]-7'-carbonyl)-L-alanine ( 2 )
[0211]
[0212] first step
[0213] (6'-Hydroxy-8'-oxo-8'H-spiro[cyclopropane-1,5'-indolezine]-7'-carbonyl)-L-alanine ( 2 )
[0214]
[0215] compound 1c (124 mg), L-alanine (250 mg) and 0.5M sodium methoxide methanol solution (5 ml); spin dry; then add n-propanol (4 ml), reflux until the reaction is complete. Cool, dilute the reaction solution with water, carefully add 1M dilute hydrochloric acid aqueous solution to acidify to about pH 4; precipitate the solid; collect the solid by filtration, then wash the solid with water; then dry to obtain 98 mg of the product 2 . 1 H NMR (500MHz, dmso-d6) δ (ppm): 17.99 (1H, s), 13.14 (1H, s), 10.14 (1H, d), 7.36 (1H, s), 6.98 (1H, s), 6.46 (1H, s), 4.53-4.46 (1H, m), 1.89 (2H, s), 1.79 (2H, s), 1.44 (3H, d). LCMS ESI(+):291(M+1) + .
Embodiment 3
[0217] (6'-Hydroxy-8'-oxo-8'H-spiro[cyclopropane-1,5'-indolezine]-7'-carbonyl)-D-alanine ( 3 )
[0218]
[0219] first step
[0220] (6'-Hydroxy-8'-oxo-8'H-spiro[cyclopropane-1,5'-indolezine]-7'-carbonyl)-D-alanine ( 3 )
[0221]
[0222] compound 1c (90 mg), D-alanine (192 mg) and 0.5M sodium methoxide methanol solution (3.6 ml); spin dry; then add n-propanol (4 ml), reflux until the reaction is complete. Cool, dilute the reaction solution with water, carefully add 1M dilute hydrochloric acid aqueous solution to acidify to about pH 4; precipitate the solid; collect the solid by filtration, then wash the solid with water; then dry to obtain 98 mg of the product 3 . 1H NMR (500MHz, dmso-d6) δ (ppm): 17.99 (1H, s), 13.16 (1H, s), 10.14 (1H, d), 7.35 (1H, s), 6.98 (1H, s), 6.45 (1H,s), 4.51-4.45(1H,m), 1.88(2H,s), 1.78(2H,s), 1.43-1.42(3H,d). LCMS ESI(+):291(M+1) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com