Heterocyclic compounds and application thereof in medicine
A compound, isomer mixture technology, used in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0177] Preparation of intermediate compounds
[0178] Preparation of Intermediate I-100':
[0179]
[0180] 1-(2,3-difluoropropyl)azetidine-3-amine ( I-100’-1 )
[0181]
[0182] first step
[0183] 2-Benzyloxymethyloxirane ( I-100'-1-a )
[0184]
[0185] NaOH (3.7 g), tetra-n-butylammonium iodide TBAI (3.43 g) and DMF (150 ml) were added to the reaction flask, cooled in an ice-water bath, and then oxirane-2-ylmethanol (CAS: 556- 52-5; 6.85 g) was dropped into the reaction flask and stirred for 2 hours; then benzyl bromide BnBr (17.3 g) was slowly added dropwise; after the addition, the reaction was left overnight at room temperature. Then, add water to quench the reaction, extract with ethyl acetate; collect the ethyl acetate layer, then wash twice with dilute sodium chloride aqueous solution, dry over anhydrous sodium sulfate, filter, and concentrate; the residue is purified by silica gel column chromatography to obtain oily product, I-100'-1-a (12 grams...
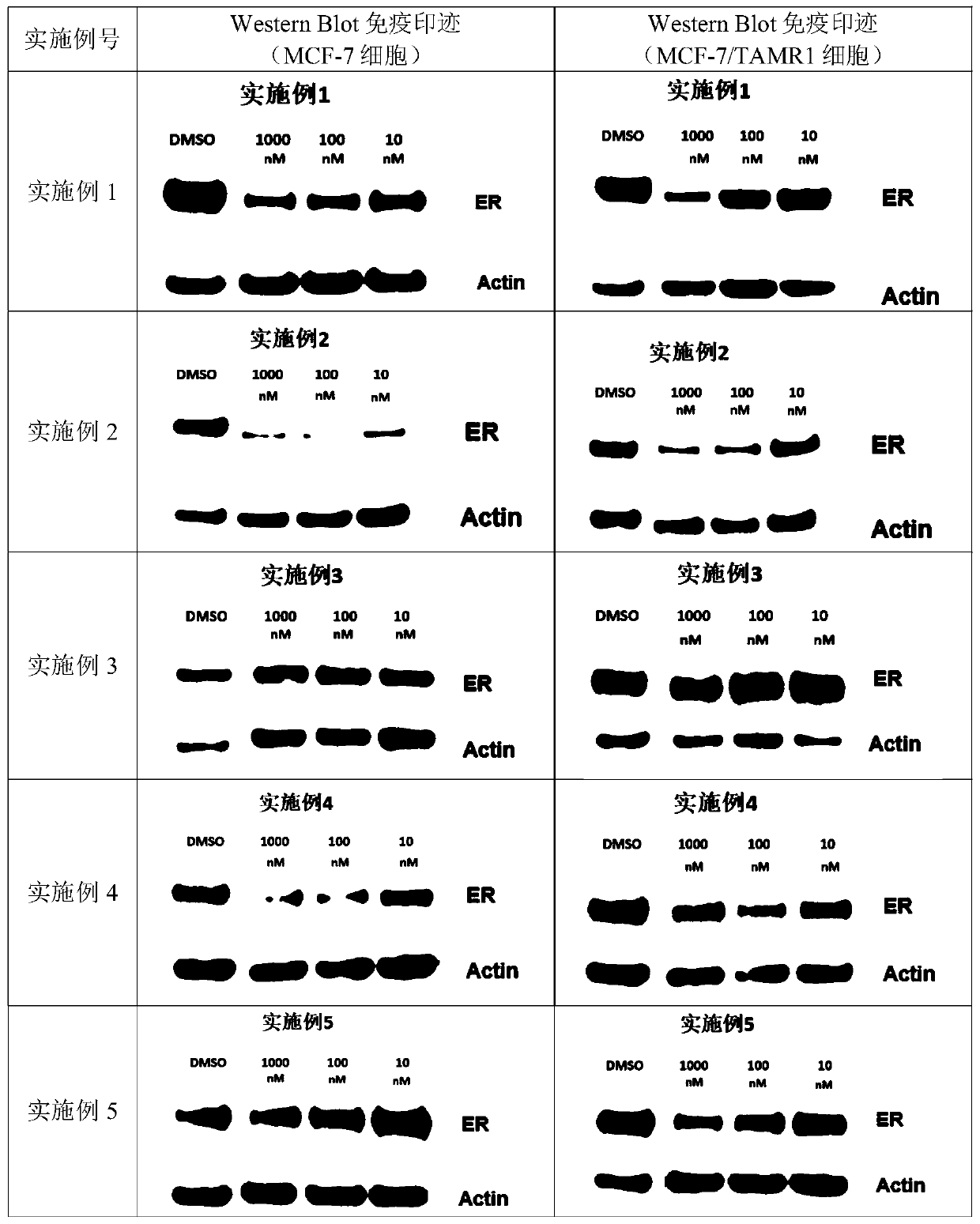
Embodiment 1
[0260] 1-(3-fluoropropyl)-N-(4-((6R,7S)-7-isobutyl-8-methyl-6,7,8,9-tetrahydro-3H-pyrazolo [3,4-h]isoquinolin-6-yl)phenyl)azetidine-3-amine ( 1 )
[0261]
[0262] first step
[0263] (2S)-2-(((1H-indazol-4-yl)methyl)(methyl)amino)-1-(4-bromophenyl)-4-methylpentan-1-ol ( 1a )
[0264]
[0265] compound I-60-1 (839 mg), 4-(chloromethyl)-1H-indazole (ADV947321888 purchased from Advanced ChemBlocks Inc., 739 mg), potassium carbonate (1.8 g) and sodium iodide (39 mg) were mixed in an water in DMF. The reaction was stirred overnight at room temperature. Then, add ethyl acetate and water for extraction; separate the organic phase, wash the organic phase twice with dilute sodium chloride aqueous solution, dry over anhydrous sodium sulfate, filter, and concentrate; the residue is purified by silica gel column chromatography to obtain a yellow solid product 1a (950 mg). 1 H NMR (500MHz, DMSO-d6) δ (ppm): 13.00 (1H, s), 8.07 (1H, s), 7.50 (2H, d, J = 8.0Hz), 7.40 (1H, d, J...
Embodiment 2
[0287] N-(3,5-difluoro-4-((6S,7S)-7-isobutyl-8-methyl-6,7,8,9-tetrahydro-3H-pyrazolo[3,4 -h] isoquinolin-6-yl)phenyl)-1-(3-fluoropropyl)azetidine-3-amine ( 2 )
[0288]
[0289] first step
[0290] (2S)-2-(((1H-indazol-4-yl)methyl)(methyl)amino)-1-(4-chloro-2,6-difluorophenyl)-4-methylpentane -1-ol ( 2a )
[0291]
[0292] compound I-60-2 (1130 mg), 4-(chloromethyl)-1H-indazole (ADV947321888 purchased from Advanced ChemBlocks Inc., 630 mg), potassium carbonate (2.5 g) and sodium iodide (54 mg) were mixed at room temperature in an water in DMF. The reaction was stirred overnight at room temperature. Then, add ethyl acetate and water for extraction; separate the organic phase, wash the organic phase twice with dilute sodium chloride aqueous solution, dry over anhydrous sodium sulfate, filter, and concentrate; the residue is purified by silica gel column chromatography to obtain a yellow solid product 2a (1660 mg). 1 H NMR (500MHz, dmso-d6) δ (in ppm): 12.98 (1H, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com