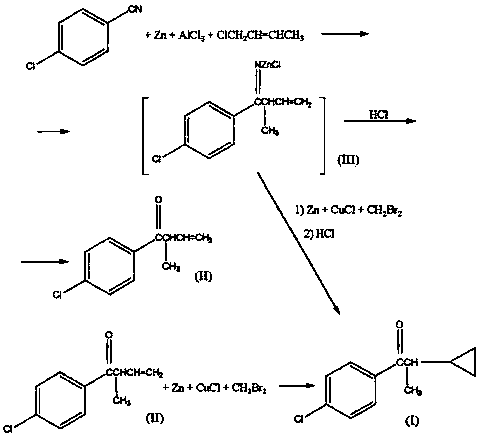
Synthesis method of 1-(4-chlorphenyl)-2-cyclopropyl-1-acetone
A synthetic method and cyclopropyl technology, applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of low product purity, long reaction route, and many side reactions, and achieve high purity, The effect of high yield and reaction conversion rate and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
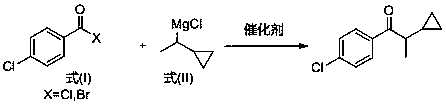
[0028] Add excess magnesium chips and 1000mL tetrahydrofuran to a dry round-bottomed flask, then dropwise add 1-chloroethylcyclopropane (1mol), the resulting mixture is stirred and reacted at room temperature for 3 hours, and filtered after reaction to remove magnesium chips to obtain Tetrahydrofuran solution (1mol / L) of the compound of formula (II); the reaction formula is as follows:
[0029]
Embodiment 2
[0031] Add 4-chlorobenzoyl chloride (0.10mol), catalyst iron triacetylacetonate (0.003mol), and isopropyl ether (250mL) into the reaction flask, and add the compound of formula (II) prepared in Example 1 dropwise at 0°C. The tetrahydrofuran solution of the compound was 100mL, after dropping, the reaction was continued at this temperature for 1 hr, after the reaction, 250mL of cold water was added dropwise to the reaction solution, after the dropping, the solid was removed by filtration, the organic phase was collected, dried, concentrated and crystallized under reduced pressure to obtain 1-( 17.3 g of 4-chlorophenyl)-2-cyclopropyl-1-propanone product, the yield was 82.9% based on 4-chlorobenzoyl chloride, and the purity by HPLC was 99.1%.
Embodiment 3
[0033] Add 4-chlorobenzoyl chloride (0.10mol), catalyst iron triacetylacetonate (0.004mol), and tetrahydrofuran (250mL) into the reaction flask, and add the compound of formula (II) prepared in Example 1 dropwise at -10°C 100mL of tetrahydrofuran solution, after dropping, continue to react at this temperature for 2hr, after the reaction, add 250mL of cold water dropwise to the reaction solution, after dropping, remove the solid by filtration, collect the organic phase, dry, concentrate and crystallize under reduced pressure to obtain 1-(4 -Chlorophenyl)-2-cyclopropyl-1-propanone product 17.7g, the yield is 84.8% based on 4-chlorobenzoyl chloride, and the purity detected by HPLC is 99.2%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com