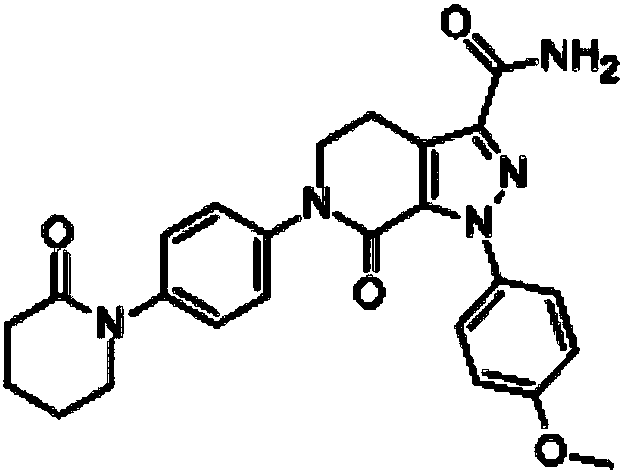
Apixaban tablets and preparation method thereof
A technology of apixaban tablets and polyvinylpyrrolidone, which is applied in the field of apixaban tablets and its preparation, can solve the problems of low bioavailability, influence of drug absorption, and low dissolution rate in vitro, and achieve increased drug Absorption, facilitate large-scale production, reduce the effect of production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
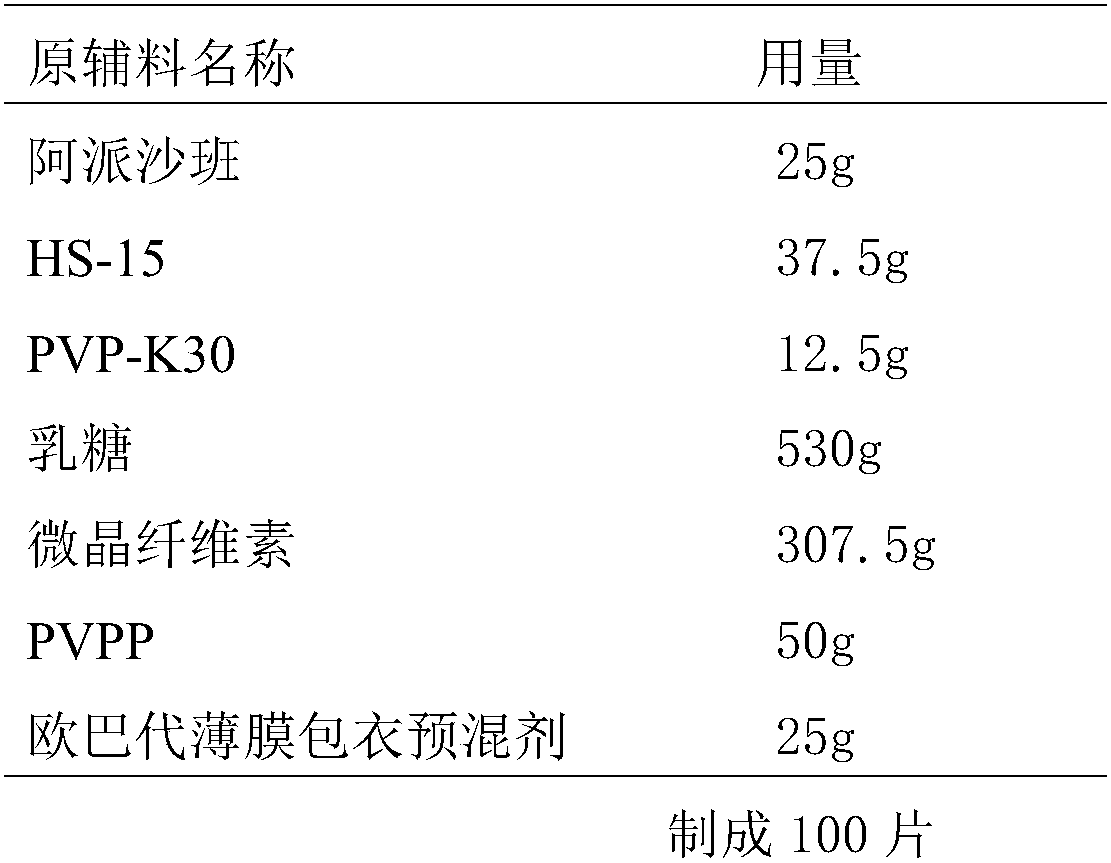
[0031] Embodiment 1, preparation Apixaban tablet
[0032] The present embodiment provides the prescription and preparation method for preparing 10000 Apixaban tablets (the specification is 2.5mg / tablet), as follows:
[0033] Prescription composition:
[0034]
[0035] The apixaban used is apixaban powder, and its particle size is controlled at D90<30um; the adopted lactose, microcrystalline cellulose and magnesium stearate are passed through 80-mesh sieves respectively for use.
[0036] The preparation method comprises the following steps:
[0037] 1) Apixaban, polyethylene glycol-12-hydroxystearate and polyvinylpyrrolidone K30 were weighed and dissolved in 2.5L ethanol, and ultrasonically assisted to dissolve to obtain a transparent solution; Spray drying removes ethanol in the transparent solution to obtain apixaban solid dispersion; the process parameters of spray drying include: air inlet temperature 120°C, air outlet temperature 80°C, air volume 0.6m 3 / min, atomiza...
Embodiment 2
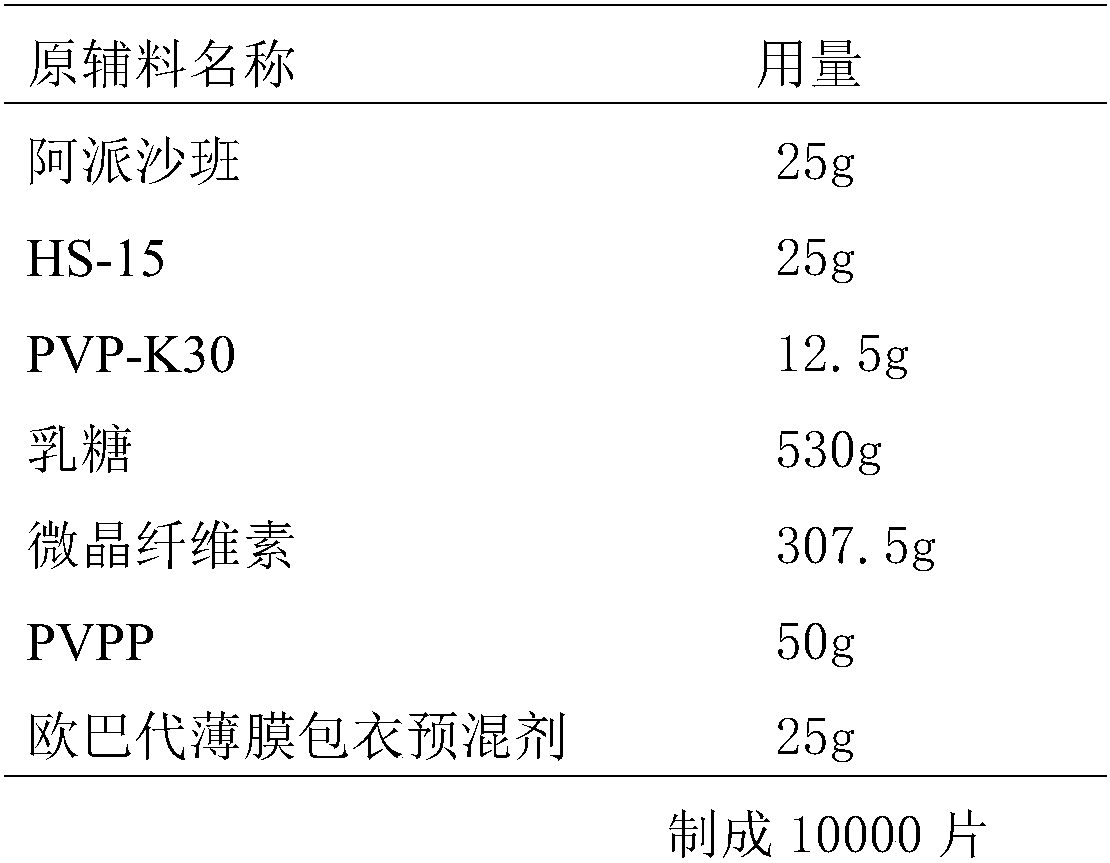
[0041] Embodiment 2, preparation Apixaban tablet
[0042] The present embodiment provides the prescription and preparation method for preparing 10000 Apixaban tablets (the specification is 2.5mg / tablet), as follows:
[0043] Prescription composition:
[0044]
[0045] The preparation method is basically the same as in Example 1.
Embodiment 3
[0046] Embodiment 3, preparation Apixaban tablet
[0047] The present embodiment provides the prescription and preparation method for preparing 10000 Apixaban tablets (the specification is 2.5mg / tablet), as follows:
[0048] Prescription composition:
[0049]
[0050]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap