A light reduction co 2 Composite photocatalyst with ultra-thin Ti-based LDHS and its preparation method
A CO2 and catalyst technology, applied in the field of visible light response photocatalyst preparation, can solve the problems of large size, small specific surface area, low catalytic activity of photocatalytic reduction, etc., and achieve the effect of high activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
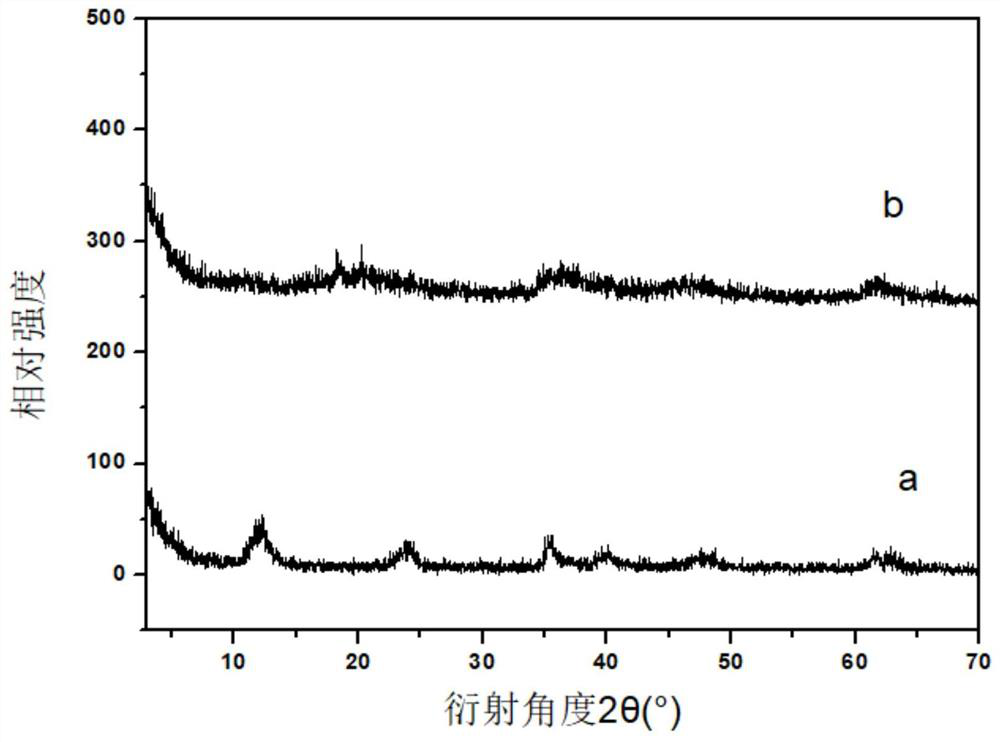
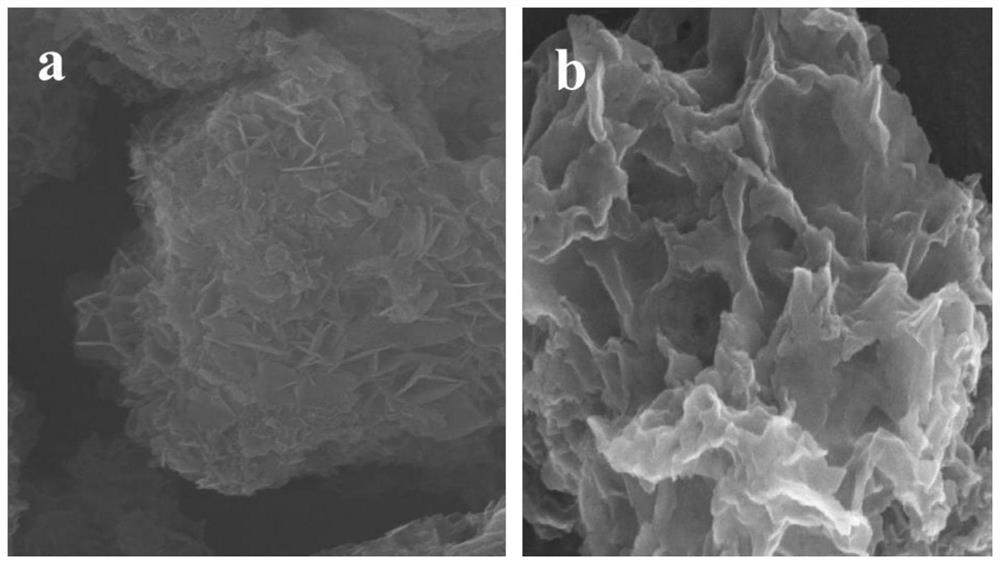
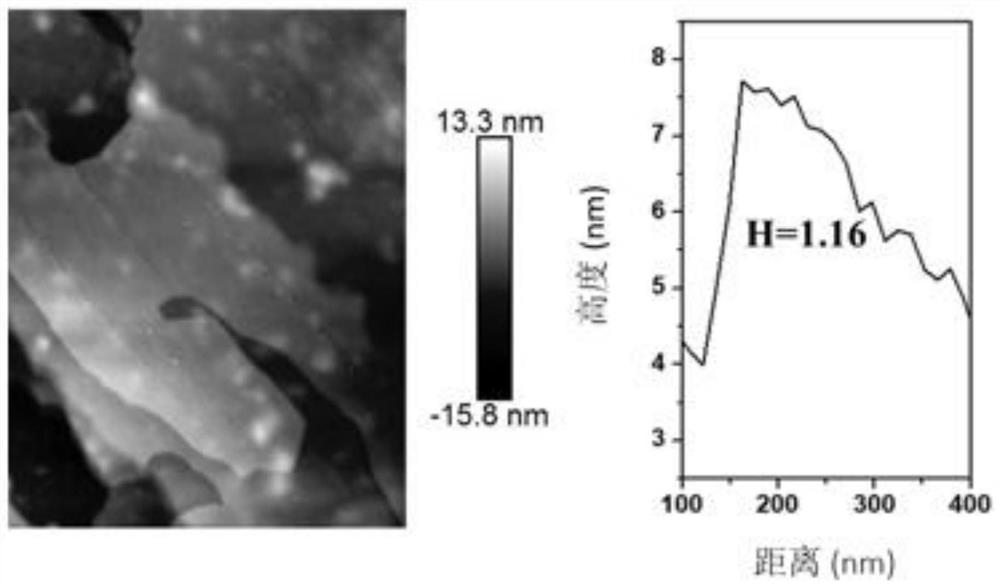
[0028] A. Measure 569 μL TiCl 4 (TiCl 4 Dissolved in concentrated HCl with a volume ratio of 1:1), weigh 0.009mol of Mg(NO 3 ) 2 ·6H 2 O, 0.006mol of Al (NO 3 ) 3 ·9H 2 O, add 150mL to remove CO 2 Dissolved in deionized water, configure mixed salt solution A; weigh 0.036mol of NaOH, add 150mL to remove CO 2 Dissolve the deionized water in the solution, configure alkaline solution B, drop solution A into the 500mL reactor at a speed of 1-10mL / min, control the dropping speed of solution B at the same time, adjust the pH of the whole system = 9-11, to be added dropwise After the end, the reaction was carried out at 80°C for 16h (the whole reaction process was 2 Completed under the protection of atmosphere), centrifuged to pH=7 of the supernatant after the reaction, and fully dried in an oven at 60 °C to obtain TiMgAl-NO 3 - -LDHs;
[0029] B. Weigh 0.2500g TiMgAl-NO of step A 3 - -LDHs was placed in a flask, 250 mL of formamide was added, and stirred for 48 h under a...
Embodiment 2
[0034] A. with embodiment 1;
[0035] B. Weigh 0.2500g TiMgAl-NO of step A 3 - -LDHs was placed in a flask, 250 mL of N,N-dimethylformamide was added, and stirred for 48 h under a nitrogen atmosphere to obtain a transparent and stable colloidal solution, expressed as U-TiMgAl-LDHs;
[0036] C. Weigh 0.0250g of C 3 N 4 Add 100 mL of an aqueous solution of sodium dodecyl sulfate with a concentration of 0.25 g / L, and ultrasonically disperse for 30 min to obtain uniformly dispersed C 3 N 4 slurry;
[0037] D. The C obtained from the C step 3 N 4 The slurry was added dropwise to the U-TiMgAl-LDHs colloidal solution in step B at a rate of 2 mL / min. After the dropwise addition was completed, stirring was continued for 30 min, alternately washed with deionized water and ethanol for several times, and vacuum dried at 60 °C to obtain U-TiMgAl-LDHs. TiMgAl-LDH / C 3 N 4 composite catalyst.
[0038] E. U-TiMgAl-LDH / C obtained by step D 3 N 4 Composite catalysts for photocatalyt...
Embodiment 3
[0040] A. Measure 569 μL TiCl 4 (TiCl 4 Dissolved in concentrated HCl with a volume ratio of 1:1), weigh 0.009mol of Zn(NO 3 ) 2 ·6H 2 O, 0.006mol of Al (NO 3 ) 3 ·9H 2 O, add 150mL to remove CO 2 Dissolved in deionized water, configure mixed salt solution A; weigh 0.036mol of NaOH, add 150mL to remove CO 2 Dissolve the deionized water in the solution, configure alkaline solution B, drop solution A into the 500mL reactor at a speed of 1-10mL / min, control the dropping speed of solution B at the same time, adjust the pH of the whole system = 9-11, to be added dropwise After the end, the reaction was carried out at 80°C for 16h (the whole reaction process was 2 Completed under the protection of atmosphere), centrifuged to pH=7 of the supernatant after the reaction, and fully dried in a 60°C oven to obtain TiZnAl-NO 3 - -LDHs;
[0041] B. Weigh 0.2500g TiZnAl-NO of step A 3 --LDHs in the flask, add 250mL formamide, stir for 48h under nitrogen atmosphere, get a transpa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com