T cell vaccine constructed by secretory component of gene engineering-based aAPC (artificial Antigen Presenting Cell) as well as preparation method and application thereof
A technology of genetic engineering and cell secretion, which is applied in the field of preparation of T cell vaccines and can solve the problems of lack of endogenous expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
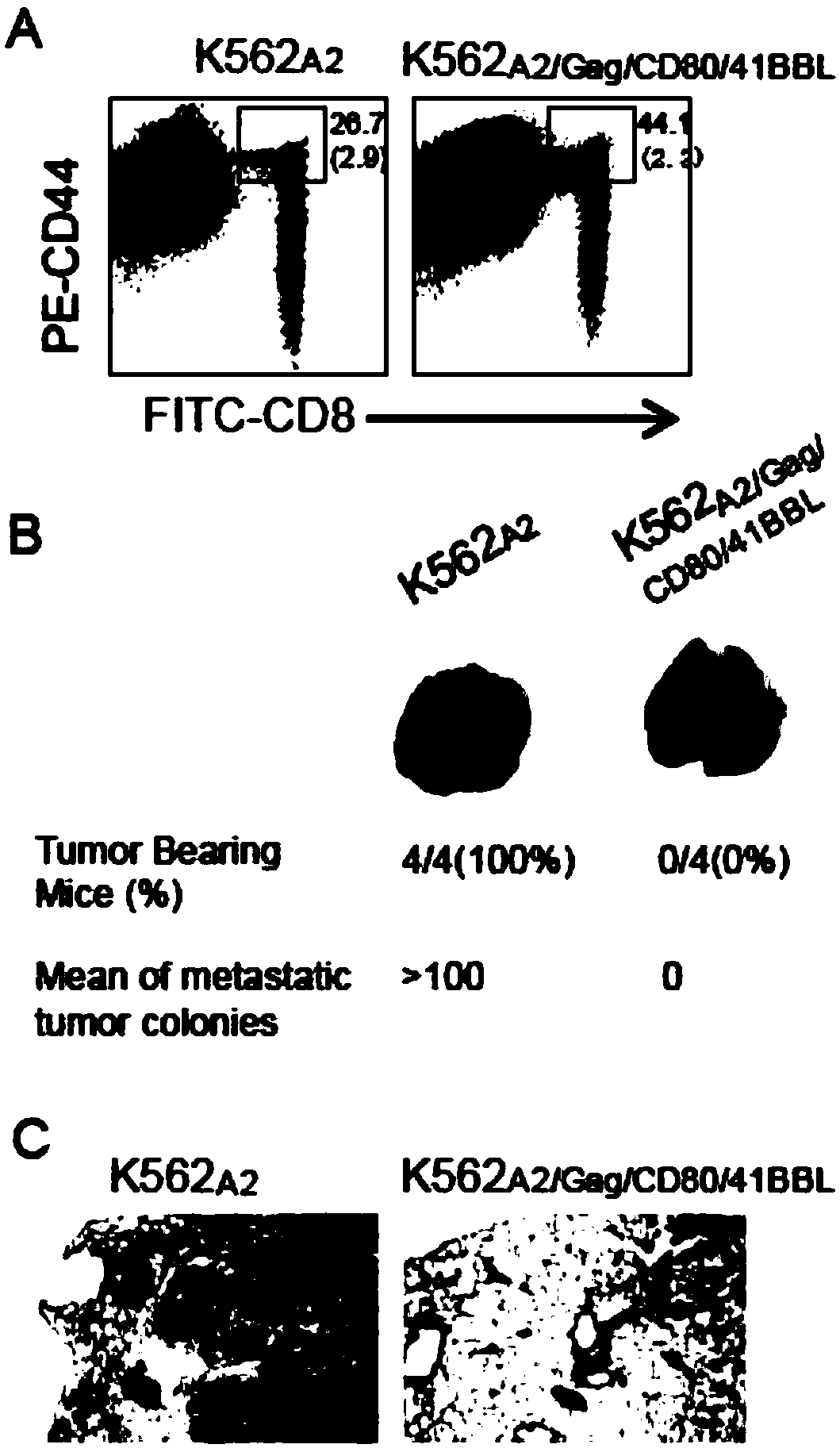
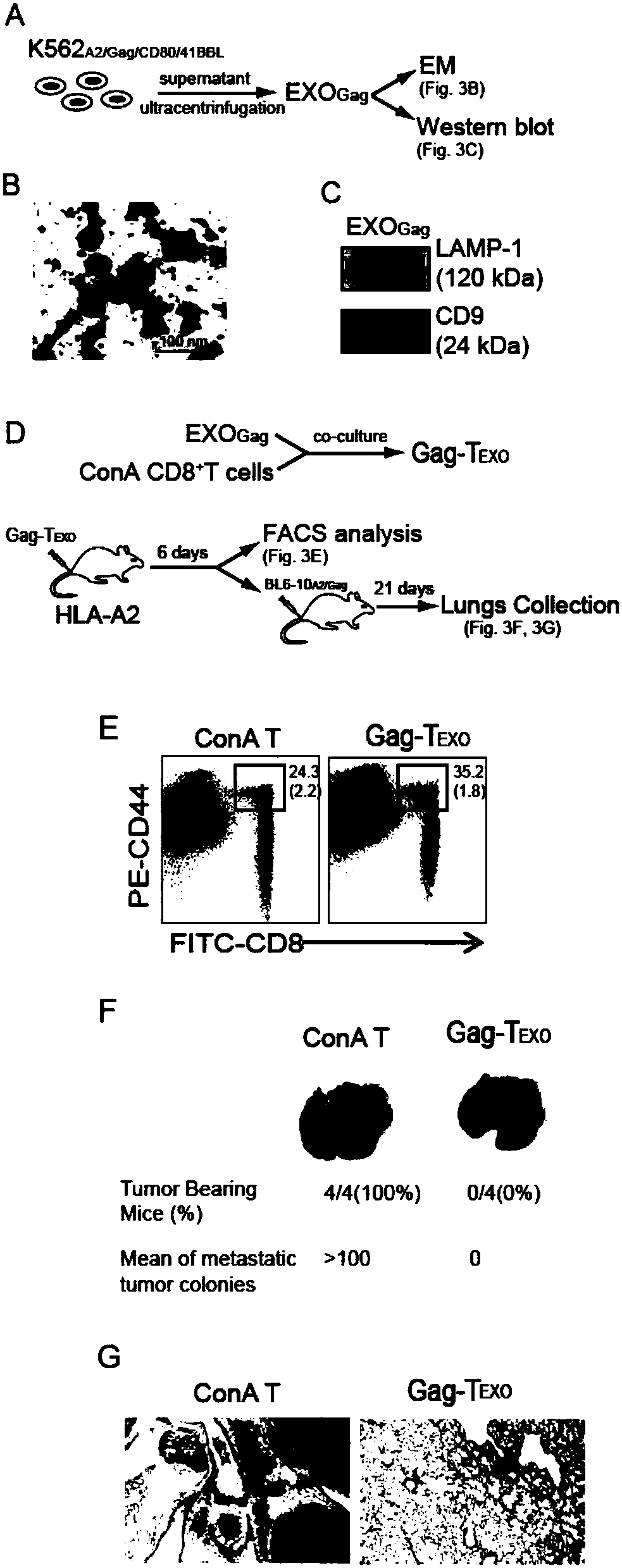
[0039] The present invention shows that activated non-specific T cells can acquire vesicles derived from genetically engineered artificial antigen-presenting cells, in particular, these cells can acquire antigen-specific histocompatibility complexes and co-stimulatory molecules from the vesicles. The present invention shows that these vesicle-derived molecules are functional. In this way, non-specific T cells targeted by vesicles can directly stimulate antigen-specific immune responses.
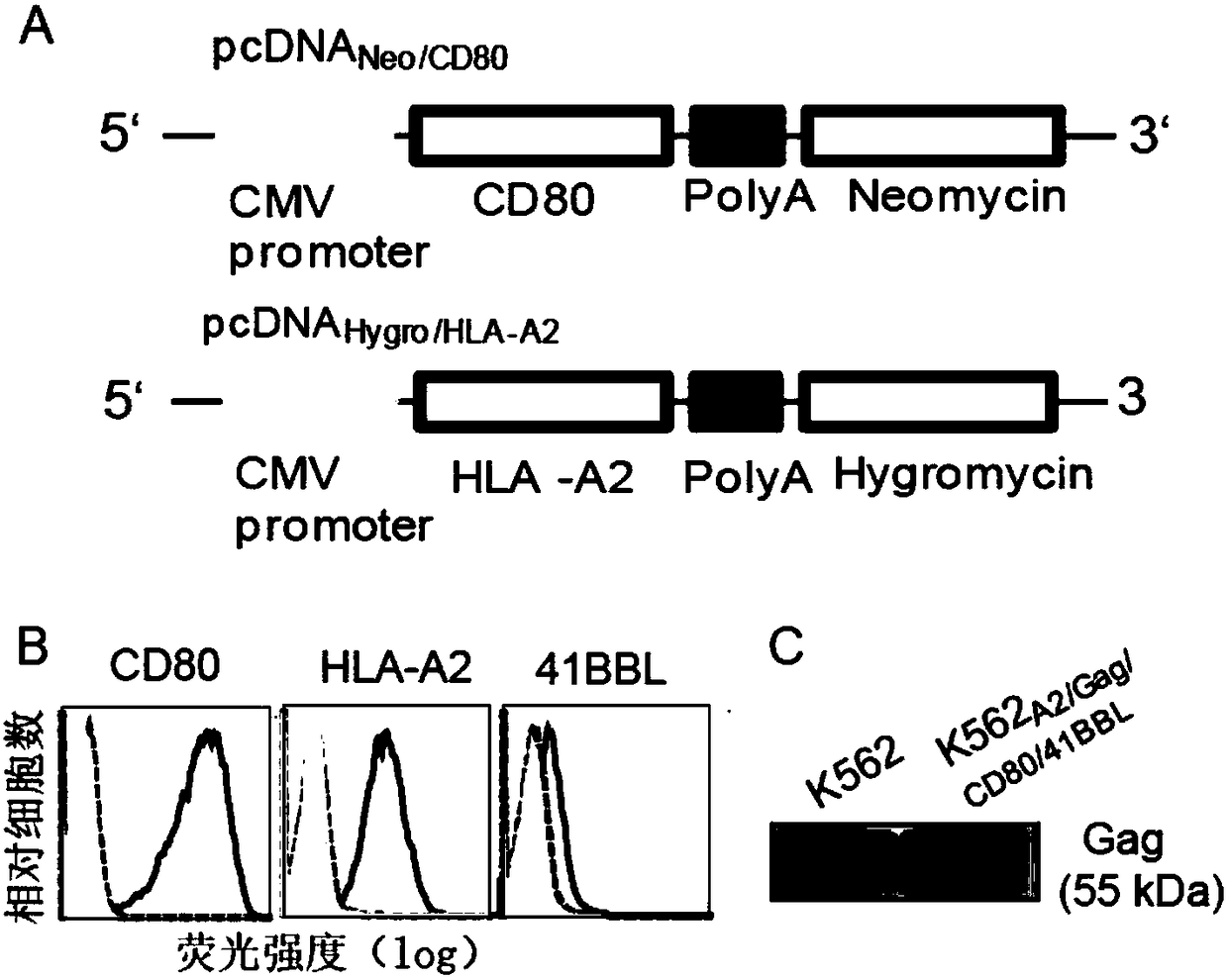
[0040] The present invention shows that the genetically engineered artificial antigen-presenting cells are derived from K562 cells that lack endogenously expressed HLA-A, B and DR histocompatibility conformers. K562 cells were first expressed by two eukaryotic expression plasmid vectors pcDNA Hygro / HLA-A2 and
[0041] pcDNA Neo / CD80 transfection. AdV Gag and AdV 41BBL Transfection to form genetically engineered antigen-presenting cells K562 expressing transgenic HLA-A2, CD80, Gag and 41...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com