Recombinant vector capable of improving solubility of viral glycoprotein, preparation method and applications thereofthereof
A technology for recombinant vectors and glycoproteins, which is applied in the fields of biotechnology and biomedicine, and can solve problems such as increasing the difficulty of protein purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0042] Implementation Case 1: Optimization of M protein and RSV pre F protein gene sequences of PIV3
[0043] The M protein of PIV3 and the RSV pre F protein gene sequence reported in the literature were retrieved from Genebank, and the kozak sequence was added in front of the promoter. Optimized according to the host cell codon preference of the Spodoptera frugiperda (Sf9) cell line, the M protein and RSV pre F protein gene sequences of the optimized PIV3 were obtained: respectively shown in SEQ ID NO: 1 and SEQ ID NO: 2 Show; in order to facilitate the purification of pre F protein with His sequence.
Embodiment example 2
[0044] Implementation case 2: Obtaining the M protein and RSV pre F protein gene sequence shuttle vector with PIV3
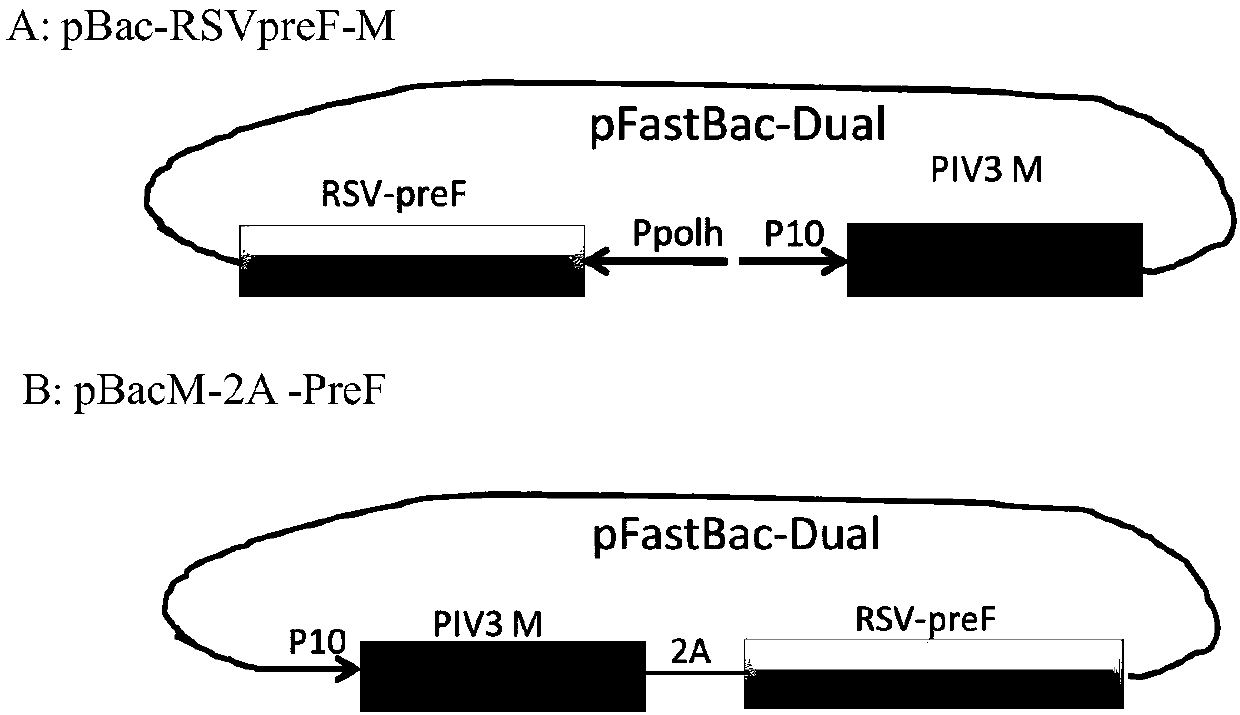
[0045] The present invention requires the co-expression of the viral M protein and the RSV preF protein, so it is necessary to construct polycistrons to meet the purpose. The current strategy for constructing polycistronic vectors mainly includes: 1) co-transfection of multiple vectors; 2) construction of multi-promoter expression vectors; 3) construction of polycistronic expression vectors using the IRES system; 4) use of self-cleaving polypeptides Ligation connects the gene of interest. Such as 2A sequence, LP4 sequence, Nia protease recognition sequence, etc. The construction of the co-expression vector of the virus M protein and the RSV pre F protein in the present invention includes but is not limited to the above methods.
[0046] Method 1: After amplifying the M protein and RSV pre F protein gene sequences of PIV3 by designing primers with restriction s...
Embodiment example 3
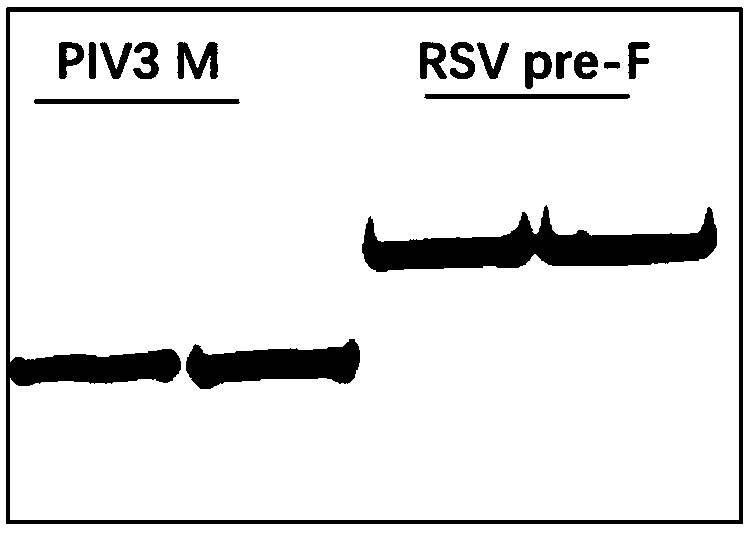
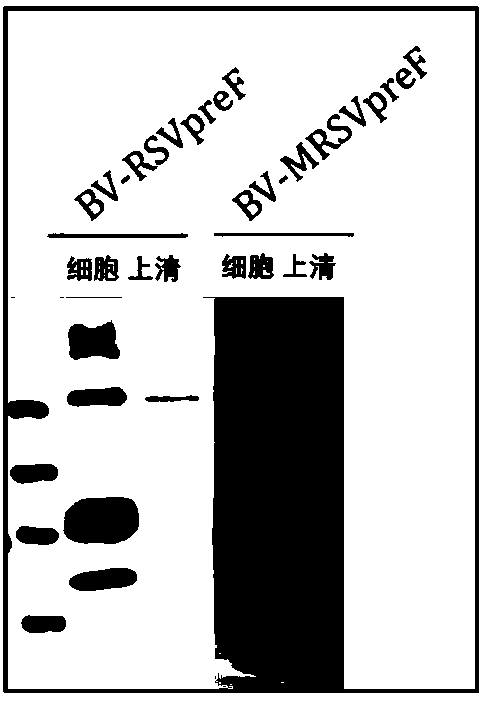
[0049] Example 3: Packaging of recombinant baculovirus expressing soluble RSV pre F protein
[0050] The recombinant baculovirus vector obtained in Example 2 was transfected into Sf9 cells, and the recombinant baculovirus containing and expressing soluble RSV pre F protein was packaged in the cell line, which were named BV-MRSVpreF and BV-M2ApreF respectively, and PCR It was determined that two recombinant baculoviruses contained two genes ( figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com