Novel 2'-uridine azide and synthetic method thereof
A technology for uridine nucleoside and synthesis method, which is applied in the field of novel 2'-azidouridine nucleoside and its synthesis, can solve the problems of short half-life, high toxicity, limited clinical application and the like, and achieves simple post-processing and reaction conditions. Gentle, easy-to-use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
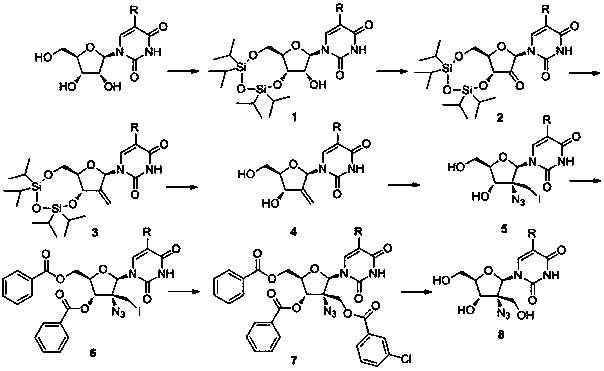
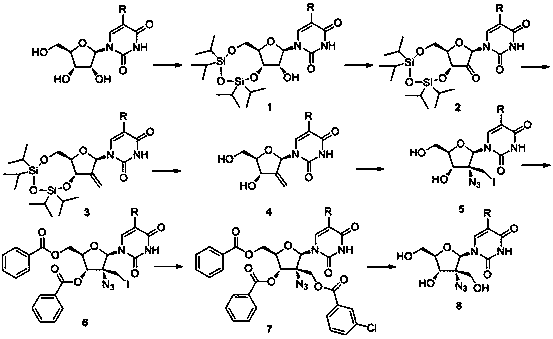
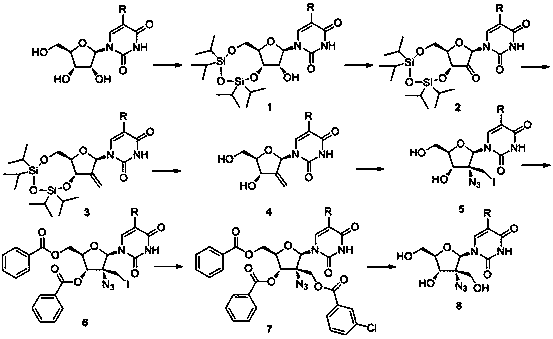
[0029] Synthesis of 3',5'-O-(1,1,3,3-tetraisopropyl-1,3-disiloxane)uridine:
[0030]
[0031] In a 100ml two-necked bottle, add 40ml pyridine and 2.4g (10mmol) uridine respectively, and under nitrogen protection conditions, add dropwise 3.2g (10mmol) 1,3 dichloro-1,1,3,3-tetraisopropyl disiloxane, then stirred at room temperature for 2 days. After the completion of the reaction detected by TLC, spin dry to remove pyridine, add 50ml of dichloromethane, wash with water three times (30ml x 3), dry with anhydrous magnesium sulfate, spin dry the solvent, load the sample by dry method, and separate by column chromatography (dichloromethane) Methane / methanol=20:1). 4.4 g of 3',5'-O-(1,1,3,3-tetraisopropyl-1,3-disiloxane)uridine was obtained with a yield of 90%.
Embodiment 2
[0033] Synthesis of 3',5'-O-(1,1,3,3-tetraisopropyl-1,3-disiloxane)-2'-oxouridine:
[0034]
[0035] In a 250ml two-necked flask, add 60ml of dichloromethane, 4.5ml of pyridine, 2.7ml of acetic anhydride and 2.7g (27mmol) of chromium trioxide, slowly drop 4.9g (10mmol) of 3',5'-O-(1, 1,3,3-Tetraisopropyl-1,3-disiloxane)uridine (dissolved in 30ml of dichloromethane), then stirred at room temperature for 30 minutes, after the TLC plate detected that the reaction was complete, add 400ml of dichloro Methane, then use a sand core funnel (add 2ml thick silica gel layer) to filter, wash the silica gel layer and precipitate with 200ml dichloromethane, combine the organic phases, wash with water three times (150ml x 3), and dry with anhydrous magnesium sulfate , and the solvent was spin-dried to obtain 4.5 g 3',5'-O-(1,1,3,3-tetraisopropyl-1,3-disiloxane)-2'-oxouridine, yield 93%.
Embodiment 3
[0037] Synthesis of 3',5'-O-(1,1,3,3-tetraisopropyl-1,3-disiloxane)-2'-deoxy-2'-methyleneuridine:
[0038]
[0039]In a 100ml two-necked flask, under nitrogen protection, 45ml dimethyl sulfoxide and 1.4g 60% NaH (35mmol) were added respectively, and then heated to 65°C until the sodium hydrogen was completely dissolved. Then cool to room temperature, add 14.3g (40mmol) methyl triphenylphosphine bromide, continue stirring for 45 minutes, add 4.8g (10mmol) 3',5'-O-(1,1,3,3-tetra isopropyl-1,3-disiloxane)-2'-oxouridine, and react at 50 °C for 1 hour. Spin to dry the solvent, add 200ml of water, adjust the pH to 7 with glacial acetic acid, extract the aqueous phase three times with dichloromethane (100ml x 3), dry with anhydrous magnesium sulfate, spin to dry the solvent, dry load, and column chromatography Separation (dichloromethane / methanol=8:1). 3.2 g of 3',5'-O-(1,1,3,3-tetraisopropyl-1,3-disiloxane)-2'-deoxy-2'-methyleneuridine were obtained, yielding rate of 67%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap