Phospholipid-miscible solvent-oil sustained-release drug delivery system composition and preparation method of local anesthetic
A technology of local anesthetics and sustained-release preparations, applied in the field of pharmacy, can solve the problems of difficult absorption and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
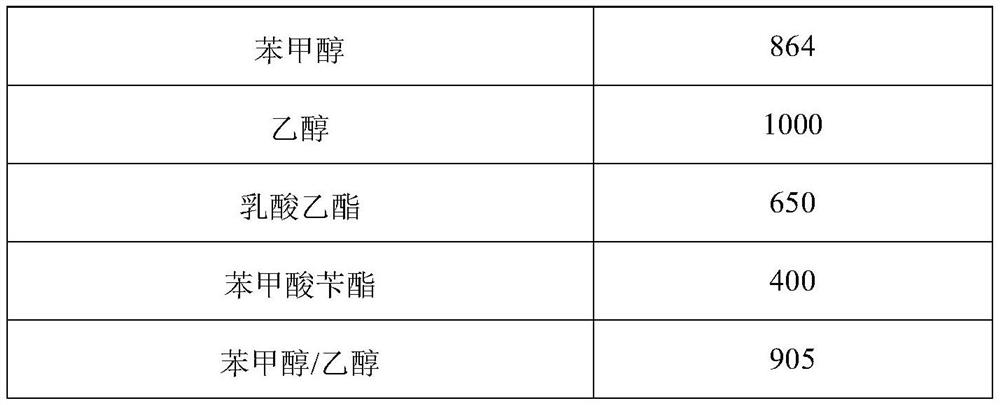
[0162] prescription:
[0163]
[0164] Preparation process: Take the prescribed amount of ethanol and benzyl alcohol, add 0.01g of ropivacaine, stir to make it fully dissolved, then add the prescribed amount of benzyl benzoate, mix well to obtain a drug solution; then add the prescribed amount of vitamin E acetate, stir to make it fully dissolved; then add the prescribed amount of egg yolk lecithin PC-98T, ultrasonically, to fully dissolve; then slowly add soybean oil to the drug solution to 10ml, and ultrasonically mix; Miscellaneous, sterilized, divided into vials, sealed, and packaged after passing the light inspection.
Embodiment 2
[0166] prescription:
[0167]
[0168]
[0169] Preparation process: Take the prescribed amount of ethanol and benzyl alcohol, put 0.8g of ropivacaine into it, stir to make it fully dissolve, then add the prescribed amount of benzyl benzoate, mix well to obtain a drug solution; then add the prescribed amount of vitamin E acetate, stir to make it fully dissolved; then add the prescribed amount of egg yolk lecithin PC-98T, ultrasonically, to fully dissolve; then slowly add soybean oil to the drug solution to 10ml, and ultrasonically mix; Miscellaneous, sterilized, divided into vials, sealed, and packaged after passing the light inspection.
Embodiment 3
[0171] prescription:
[0172]
[0173] Preparation process: take the prescribed amount of ethanol, throw 0.3g of ropivacaine, stir to make it fully dissolve, add the prescribed amount of benzyl benzoate, mix well, and obtain a drug solution; then add the prescribed amount of vitamin E acetate, Stir to make it fully dissolved; then add the prescribed amount of egg yolk lecithin PC-98T, and ultrasonically dissolve it; then slowly add soybean oil to the drug solution to 10ml, and ultrasonically mix; remove impurities and bacteria by membrane filtration , sub-packed into vials, sealed, and packaged after passing the light inspection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com