A kind of clean preparation method of layered composite metal hydroxide
A technology of hydroxide and layered compounding, applied in chemical instruments and methods, aluminum compounds, inorganic chemistry, etc., can solve the problems of high reaction temperature, waste of water resources, long reaction time, etc., achieve mild reaction conditions and save production Effects of shortening cost and response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Step A: Take 58.00g Mg(OH) 2 and 21.12 g 4MgCO 3 ·Mg(OH) 2 ·5H 2 O was mixed, placed in 800 g of deionized water, and ground and dispersed at a speed of 3000 rpm in a basket mill for 20 minutes to obtain slurry A.
[0026] Step B: Take 13.05g Al(NO 3 ) 3 9H 2 O was fully dissolved in 80mL of deionized water; 80mL of slurry A and 80ml of solution B were added to a three-necked flask with a reflux device, and the temperature was raised to 80°C under stirring conditions, and the reaction was carried out at a stirring speed of 400 rpm for 6 hours. The product obtained by centrifugation was directly dried at 60°C for 8 hours to obtain the molecular formula Mg 4 Al 2 (OH) 12 CO 3 4H 2 O's LDHs products.
[0027] Reactant 4MgCO 3 ·Mg(OH) 2 ·5H 2 O, Mg(OH) 2 , Al(NO 3 ) 3 9H 2 The molar ratio of O is 1:23:8.
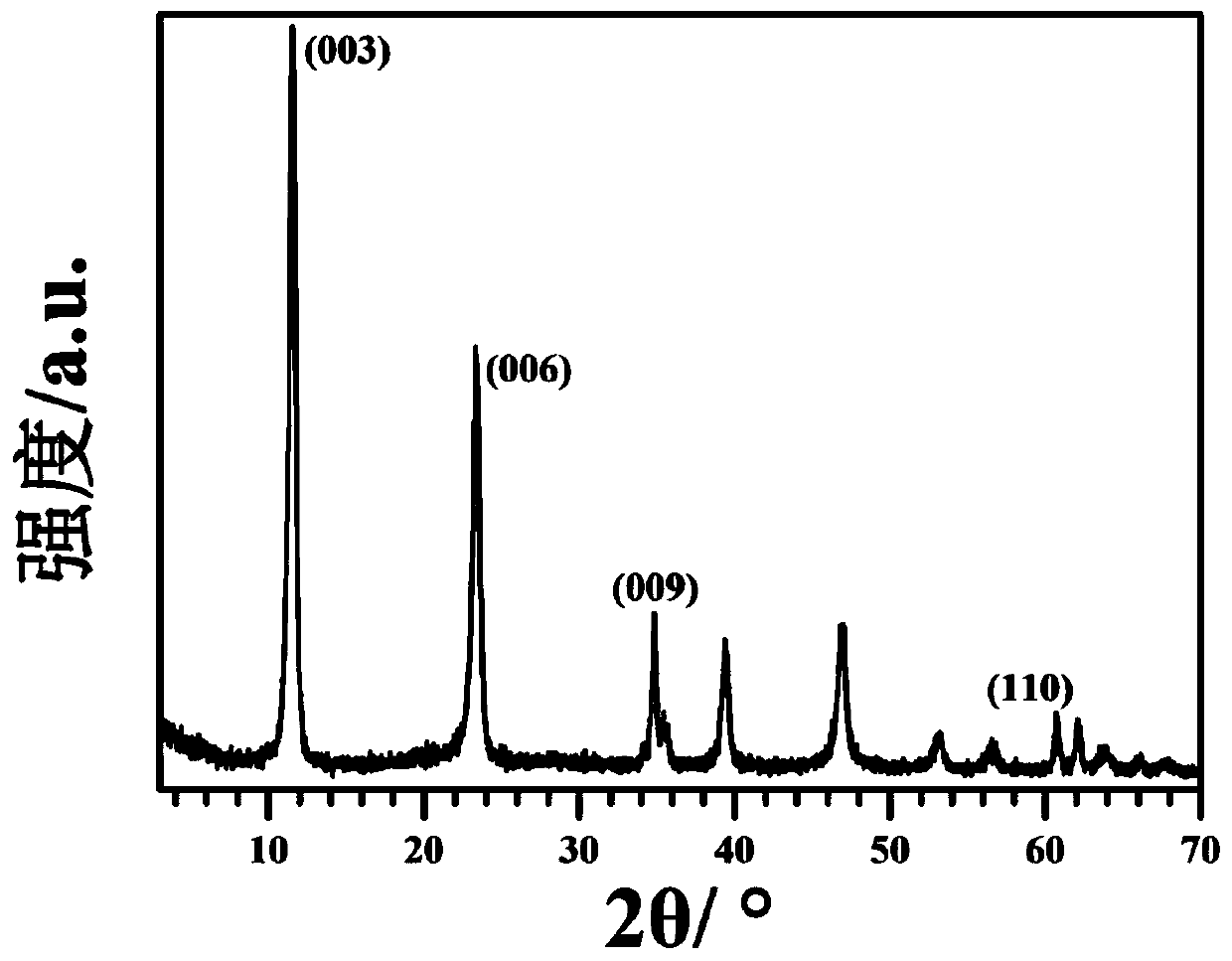
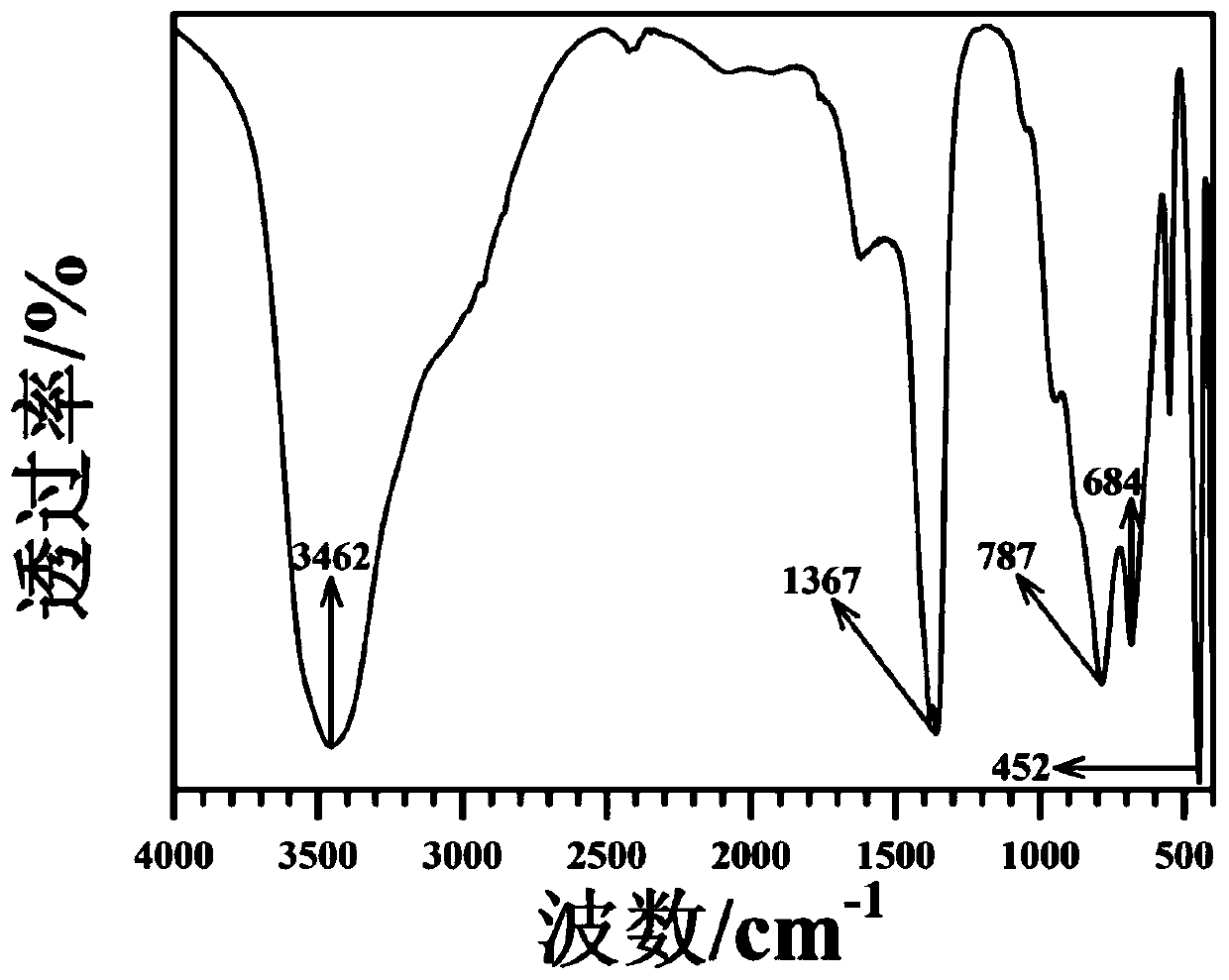
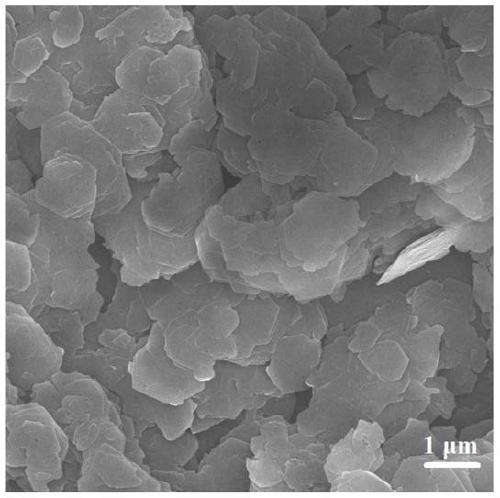
[0028] The results of elemental analysis using the Japan Shimadzu ICPS-7500 elemental analyzer show that the product contains Mg:Al=2:1, and the produc...
Embodiment 2
[0030] Step A: Take 58.00g Mg(OH) 2 and 21.12 g 4MgCO 3 ·Mg(OH) 2 ·5H 2 O mixed, placed in 800g of deionized water, added to a basket mill, and dispersed for 20 minutes at a speed of 3000 rpm to obtain slurry A.
[0031] Step B:
[0032] Take 8.40g AlCl 3 ·6H 2 O was fully dissolved in 80mL of deionized water; 80mL of slurry A and 80mL of solution B were added to a three-necked flask with a reflux device, and the temperature was raised to 80°C under stirring conditions, and the reaction was carried out at a stirring speed of 400 rpm for 6 hours. The product obtained by centrifugation was directly dried at 60°C for 8 hours to obtain the molecular formula Mg 4 Al 2 (OH) 12 CO 3 4H 2 O's LDHs products.
[0033] Reactant 4MgCO 3 ·Mg(OH) 2 ·5H 2 O, Mg(OH) 2 , AlCl 3 ·6H 2 The molar ratio of O is 1:23:8.
Embodiment 3
[0035] Step A: Take 58.00g Mg(OH) 2 and 21.12 g 4MgCO 3 ·Mg(OH) 2 ·5H 2 O mixed, placed in 800g of deionized water, added to a basket mill, and dispersed for 20 minutes at a speed of 3000 rpm to obtain slurry A.
[0036] Step B: Take 5.95g Al 2 (SO 4 ) 3 Place in 80mL deionized water to fully dissolve; take 80mL of slurry A and 80mL of solution B and add them to a three-neck flask with a reflux device, heat up to 80°C under stirring conditions, react at a stirring speed of 400 rpm for 6 hours, and centrifuge The isolated product was directly dried at 60°C for 8 hours to obtain the molecular formula Mg 4 Al 2 (OH) 12 CO 3 4H 2 O's LDHs products.
[0037] Reactant 4MgCO 3 ·Mg(OH) 2 ·5H 2 O, Mg(OH) 2 、Al 2 (SO 4 ) 3 The molar ratio is 1:23:4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com