3,3'-(3,5-difluorobenzylidene)-bis-4-hydroxycoumarin and application thereof
A technology of difluorobenzylidene and hydroxycoumarin, applied in the directions of organic chemistry, drug combination, antitumor drugs, etc., to achieve the effects of low toxicity, good anti-leukemia activity and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Synthetic route and method of the compound of the present invention
[0018]
[0019] R=H, 3,5-2F, 3,5-2Cl, 3,5-2Br, 3,5-2OCH 3 Or 3,4-2CF 3 .
[0020] resolve resolution:
[0021] Mix 20mmol of 4-hydroxycoumarin and 100mL of absolute ethanol and heat until 4-hydroxycoumarin is dissolved, add 10mmol series of aromatic aldehydes containing different substituents (the compound R=H does not have this step), and heat After refluxing, white solid particles are precipitated. Continue heating. After the reaction is over, cool and filter with suction, and then recrystallize with ethanol to finally obtain pure white granular crystals.
Embodiment 2
[0022] Example 2: Structural identification
[0023] The molecular weight, structure and purity of the synthesized series of 4-hydroxycoumarin compounds were identified by nuclear magnetic resonance spectroscopy (NMR).
[0024] Compound 1:
[0025] 3,3′-benzylidene-bis-4-hydroxycoumarin
[0026] 3,3′-Benzylidene-bis-(4-hydroxycoumarin)
[0027] 1 H NMR(CDCl 3 ,δ,ppm): 11.528(s,1H),11.299(s,1H),7.994-8.080(q,2H),7.606-7.649(m,2H),7.215-7.421(m,9H),6.104(s ,1H).
[0028] Compound 2:
[0029] 3,3′-(3,5-difluorobenzylidene)-bis-4-hydroxycoumarin
[0030] 3,3′-(3,5-Difluorobenzylidene)-bis-(4-hydroxycoumarin)
[0031] 1 H NMR(CDCl 3 ,δ,ppm): 11.666 (s, 1H), 11.323 (s, 1H), 8.033-8.109 (q, 2H), 7.660-7.701 (m, 2H), 7.427-7.462 (t, 4H), 6.731-6.790 (m,3H),6.051(s,1H).
[0032] Compound 3:
[0033] 3,3′-(3,5-Dichlorobenzylidene)-bis-4-hydroxycoumarin
[0034] 3,3′-(3,5-Dichlorobenzylidene)-bis-(4-hydroxycoumarin)
[0035] 1 H NMR(CDCl 3 ,δ,ppm): 11.630 (s, 1H), 11.314 (s, 1H), 8.04-8.114 (m, 2H), 7.6...
Embodiment 3
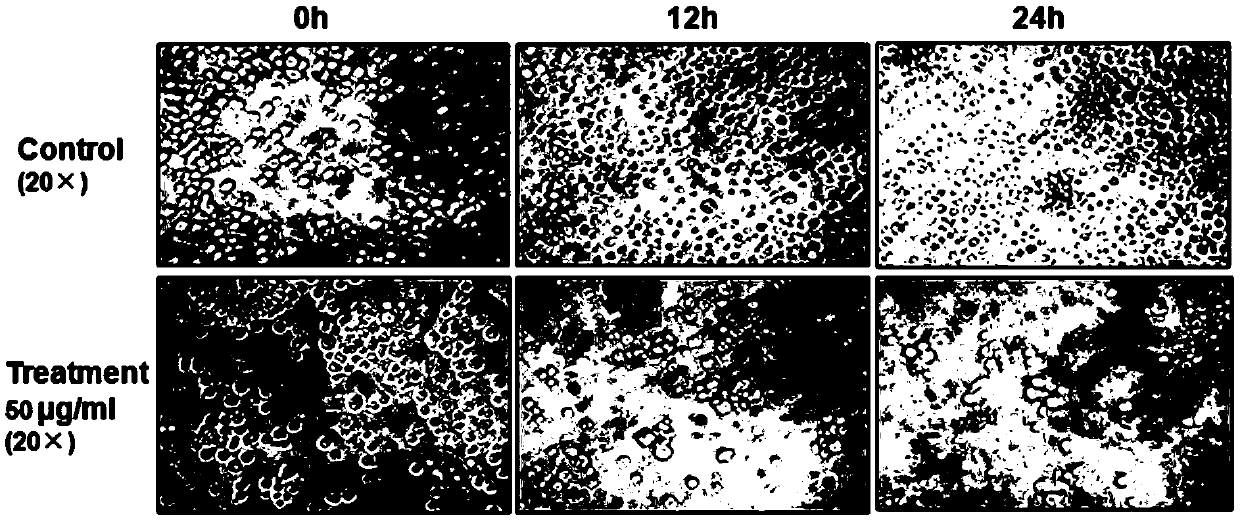
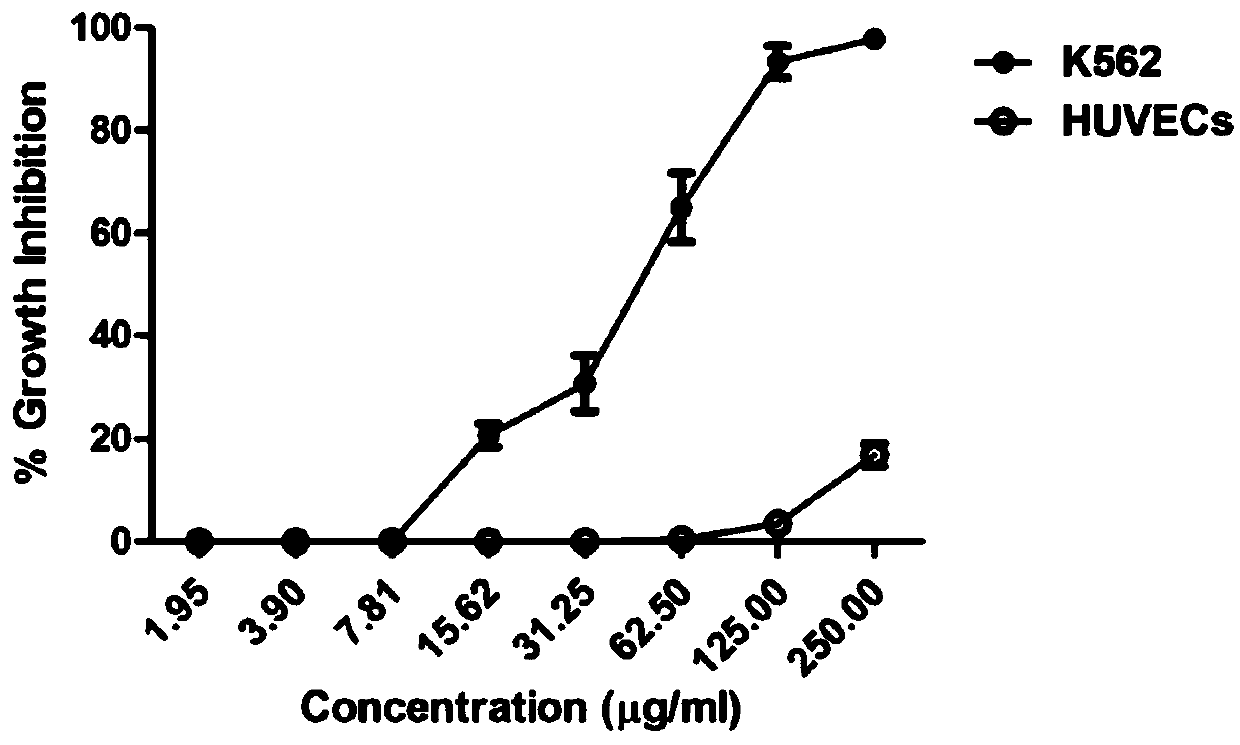
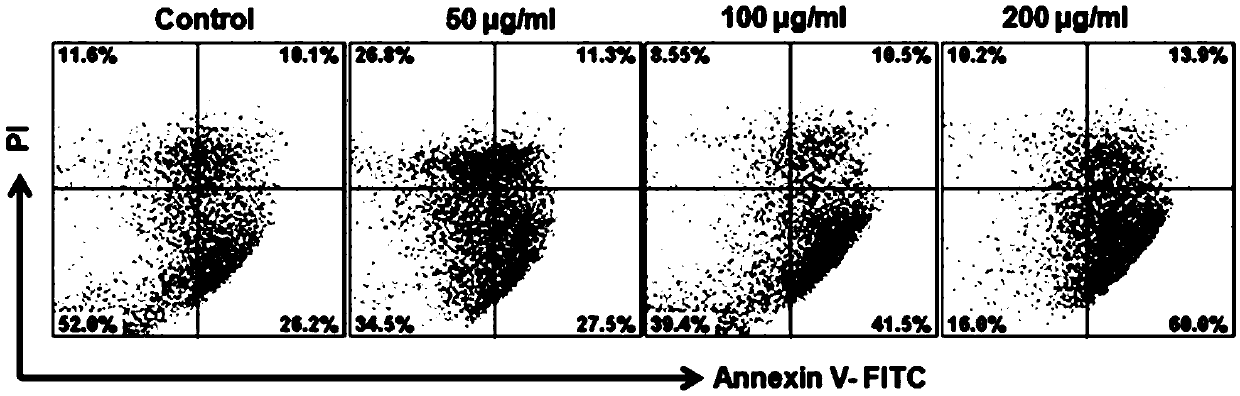
[0053] Example 3: Inhibition rate of compound 1-6 on the proliferation of human chronic myeloid leukemia cells K562 cells and human umbilical vein endothelial cells HUVECs
[0054] (1) The MTT method was used to detect the inhibitory rate of compound 1-6 on the proliferation of human chronic myeloid leukemia cells K562 cells and human umbilical vein endothelial cells HUVECs. K562 cells and HUVECs were cultured to 80% confluence for passage, with 2.0 ×10 5 Inoculated in 96-well culture plate at a density of pieces / mL, in 5% CO 2 , 37 ℃ incubator overnight. The supernatant was discarded, and the 6 compounds obtained in Example 1 were diluted with RPMI-1640 and MEM containing 2% fetal bovine serum. The above compounds were added to each well to a final concentration of 1, 2, 4, 8, 16, 32, 64, 128, 256μg / ml, set three repeat holes, and set blank holes. MTT method was used to detect cell viability in 24 hours. Cell survival rate=(A administration-A blank) / (A control-A blank). The e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com