Posterior ocular fibrosis inhibition by antagonizing placental growth factor
A technology of placental growth factor and growth factor, applied in the field of eye treatment, can solve problems such as lack of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0141] 1 Introduction
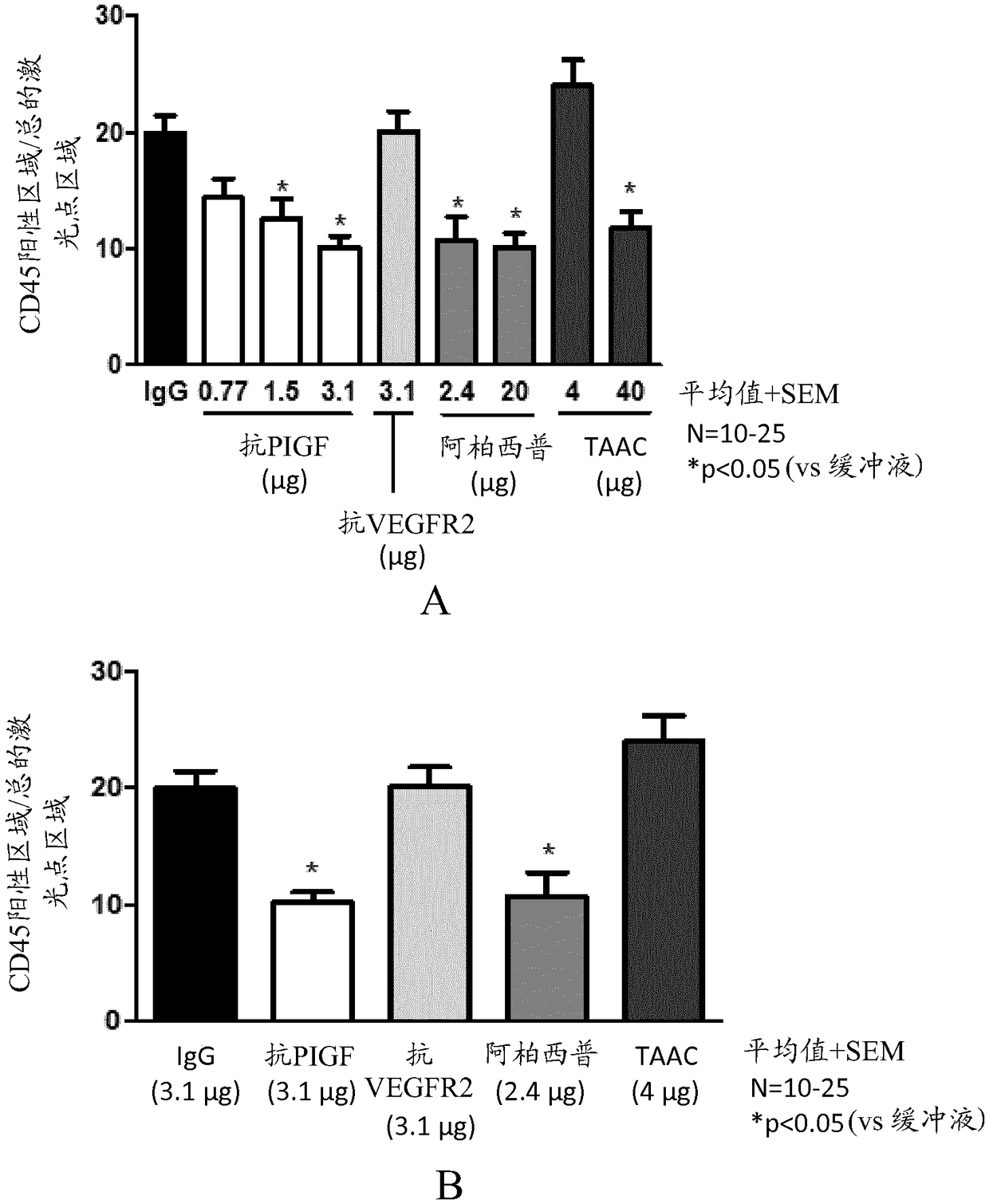
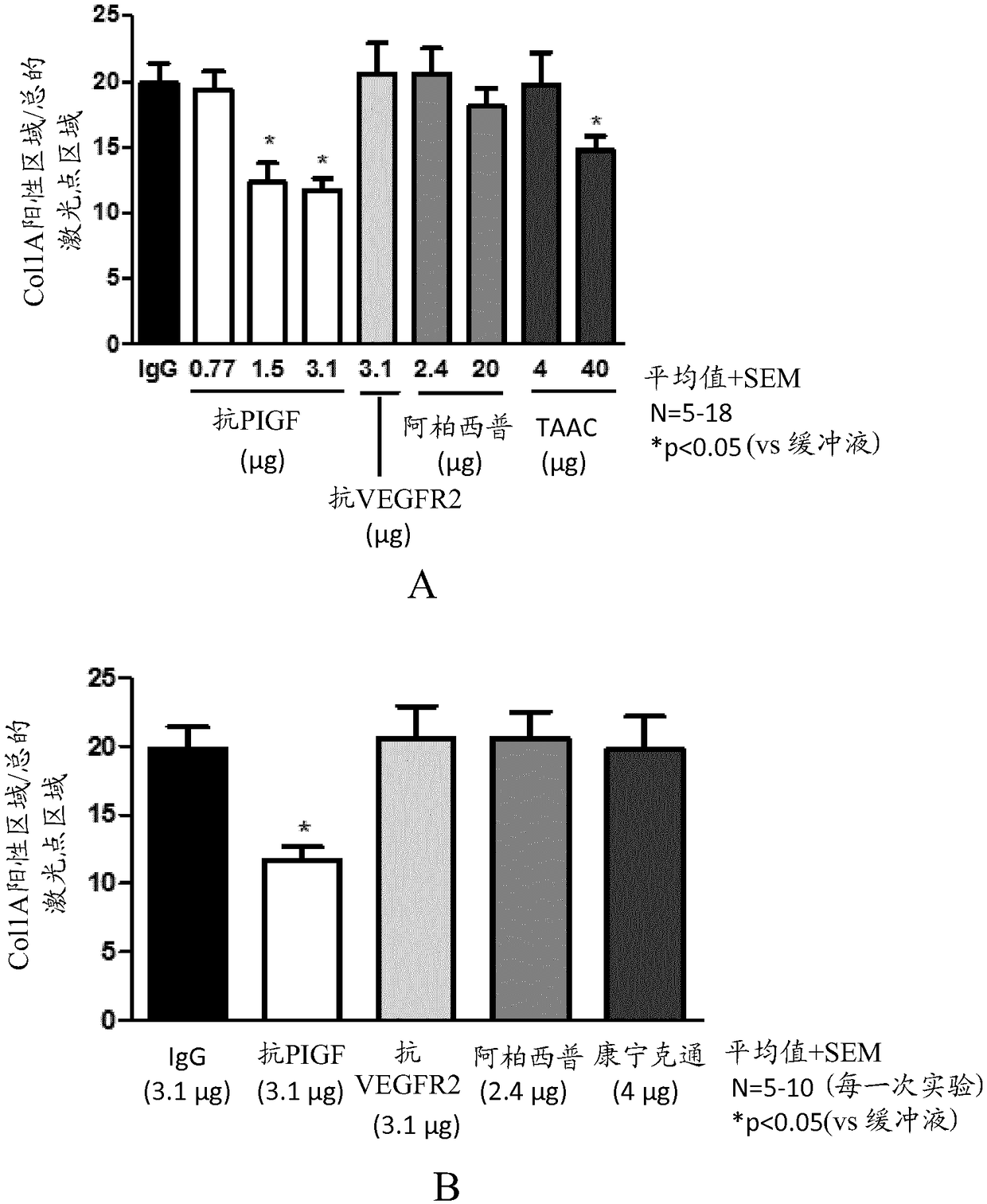
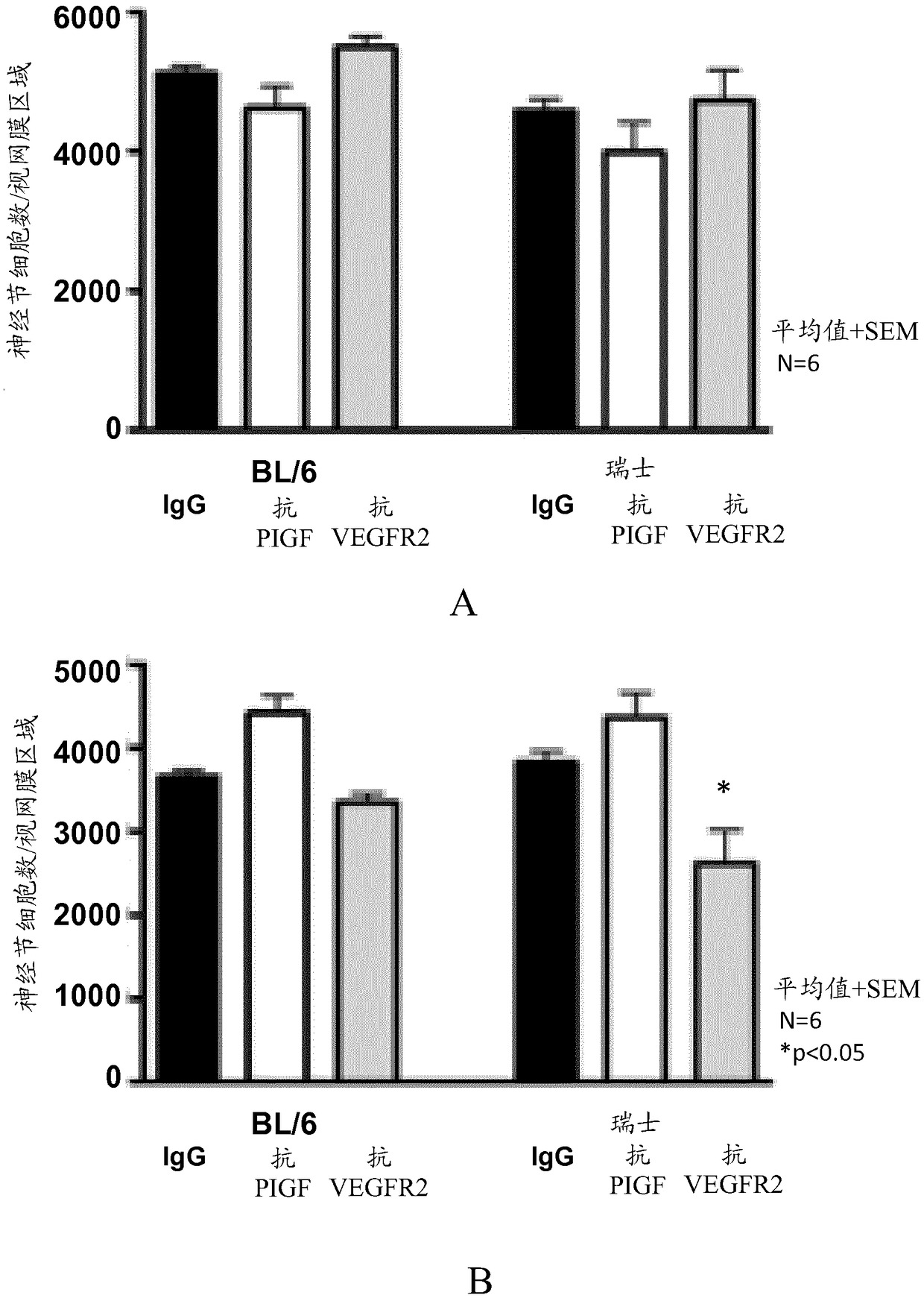
[0142] This study investigated the effect of anti-PlGF antibodies (mouse PLGF inhibitory antibody 5D11D4 or human PlGF inhibitory antibody 16D3, ThromboGenics, Leuven, Belgium) on one or more of neovascularization, inflammation and collagen deposition in a mouse model of CNV. Dose-response potency of PlGF inhibition; and comparing it with equimolar concentrations of anti-VEGF-R2 antibody (DC101, produced by hybridoma cell line ATCC HB-11534), aflibercept ( Bayer), triamcinolone acetonide (TAAC; Bristol-Myers Squibb) was compared with the anti-mouse VEGF antibody B20 (Liang et al. 2006, JBiol Chem 281:951-961). TAAC was used as a reference for inflammation and fibrosis. Based on the activity in the mouse CNV model described by Takata et al. (Takata et al., Sci Rep 2015, 5:9898), a single injection of 1 μL TAAC was chosen as the treatment regimen. The effects of anti-PlGF antibody 5D11D4 and anti-VEGF-R2 antibody DC101 on RGC survival were studied ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com