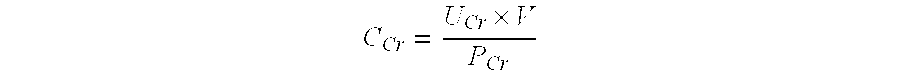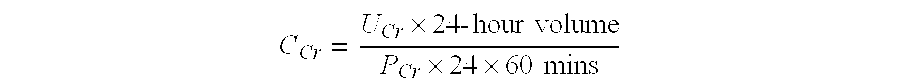Patents
Literature
45 results about "Placenta Growth Factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
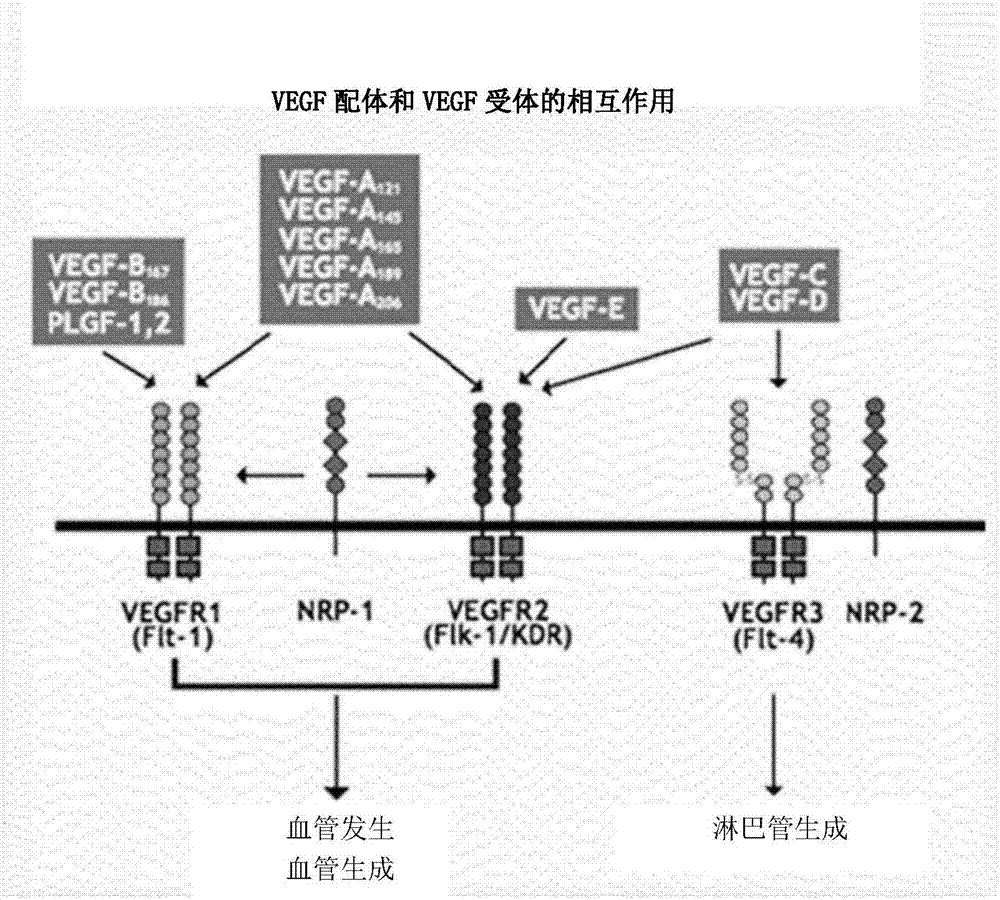
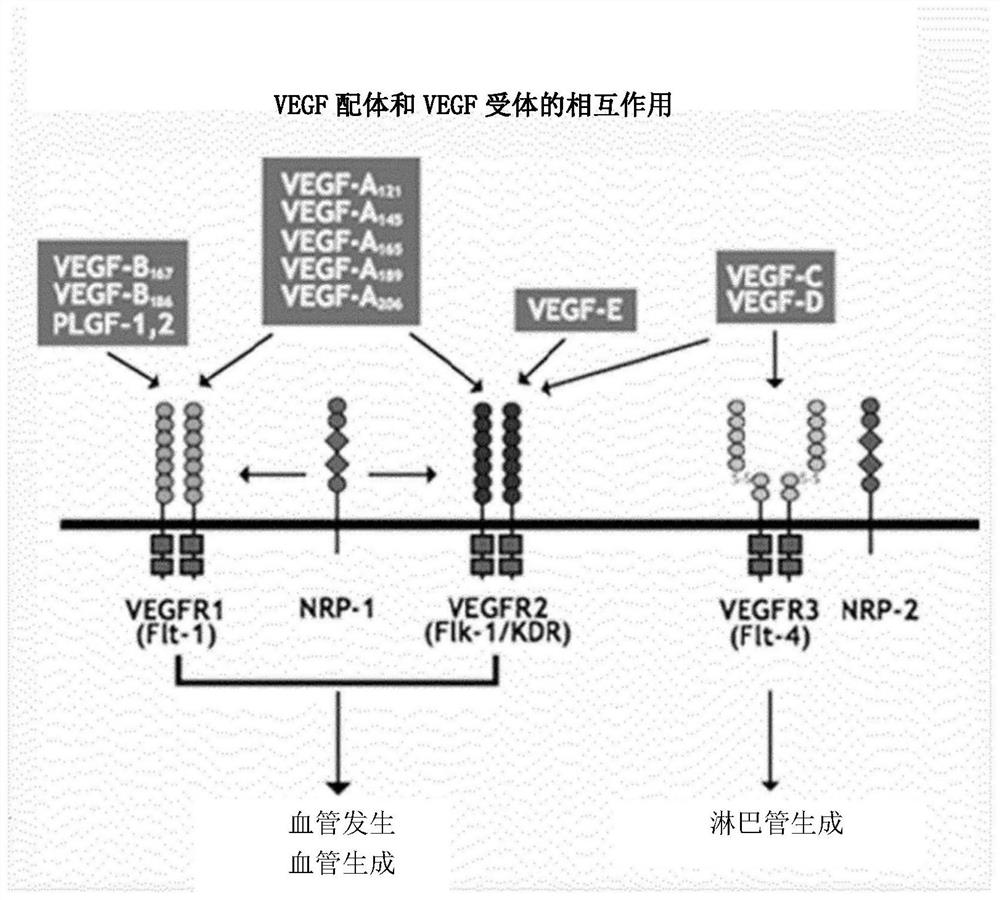
Placental growth factor is a protein that in humans is encoded by the PGF gene. Placental growth factor (PGF) is a member of the VEGF (vascular endothelial growth factor) sub-family - a key molecule in angiogenesis and vasculogenesis, in particular during embryogenesis. The main source of PGF during pregnancy is the placental trophoblast.
Method for assessment of severity of liver cirrhosis
InactiveUS20110257022A1Peptide librariesLibrary screeningHepatocyte growth factorLiver transplantation
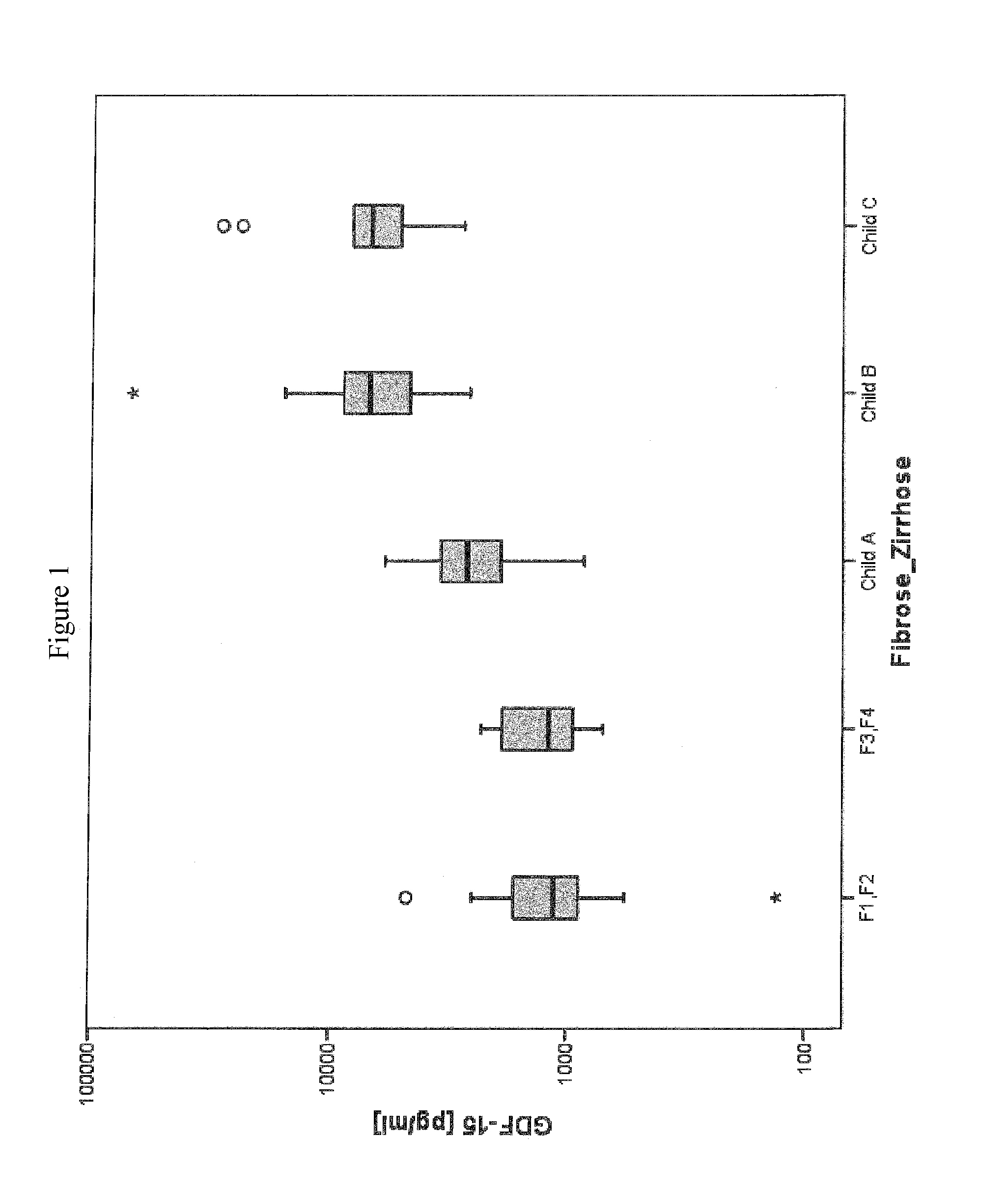
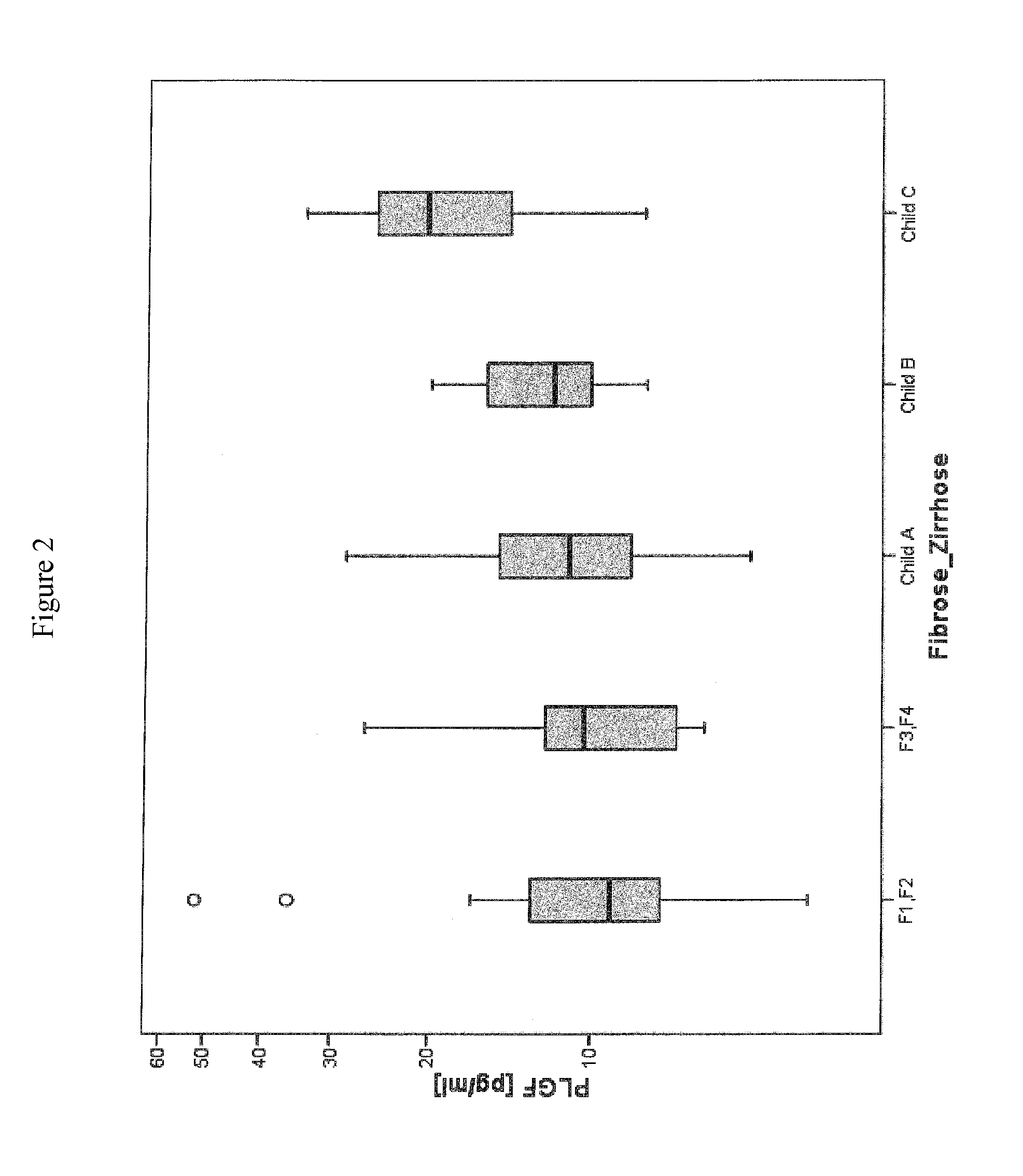
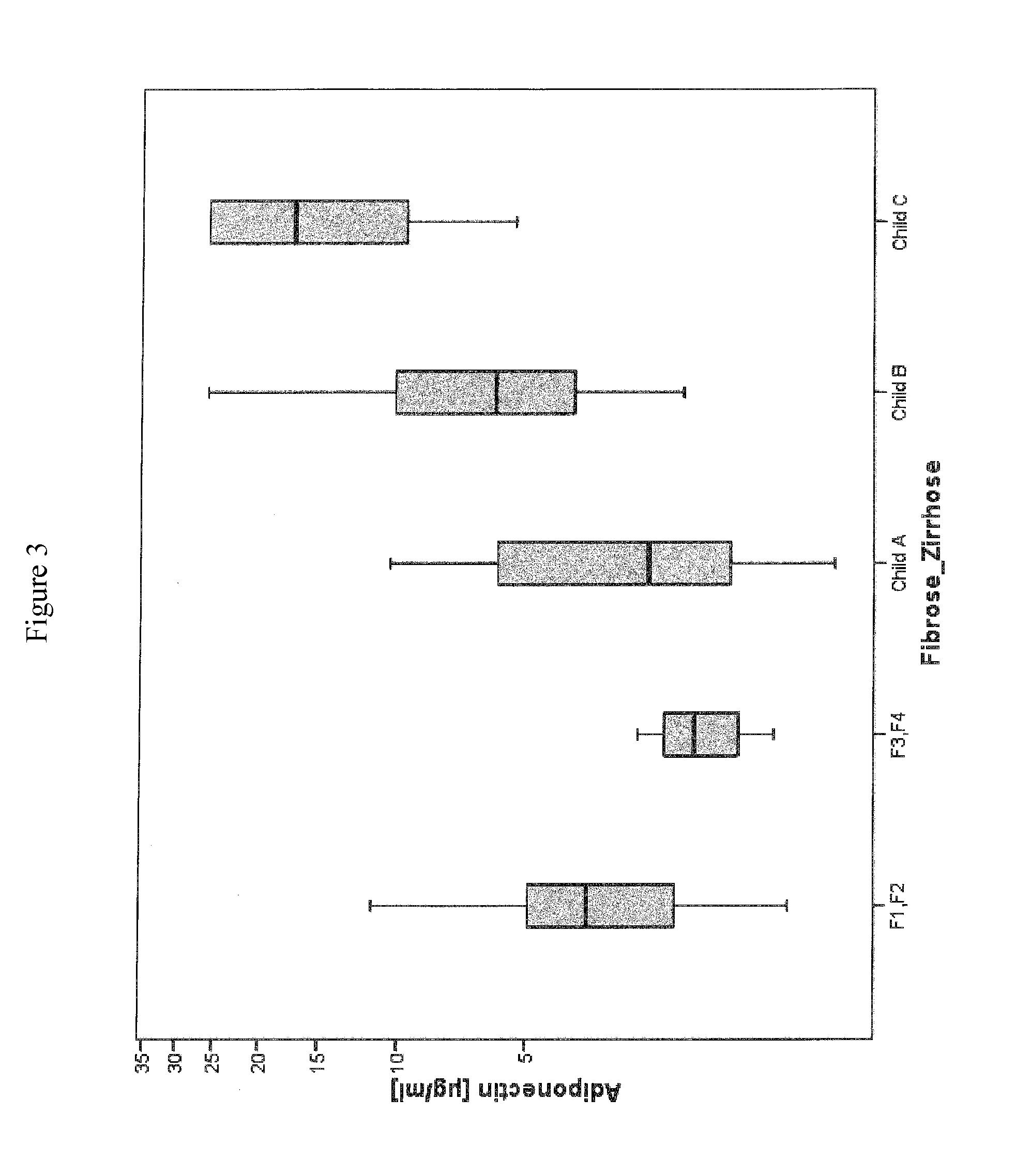
Disclosed is a method for diagnosing whether a subject suffers from a mild or severe form of liver cirrhosis based on determining the amount of GDF-15 (growth differentiation factor 15), PlGF (placental growth factor), and / or hepatocyte growth factor (HGF) in a sample from the subject and comparing the thus determined amount(s) with a reference amount (reference amounts). The method may further include determining the amount of adiponectin in a sample from the subject, and comparing the amount to a reference amount for adiponectin. Also described is a method to identifying a subject being susceptible to liver transplantation including determining the amount of GDF-15, PlGF, and / or HGF in a sample from the subject and comparing the thus determined amount(s) with a reference amount (reference amounts).
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Binding partners of the placental growth factor, especially antibodies directed against the placental growth factor, and production and use thereof
ActiveUS20090068679A1Peptide/protein ingredientsAntibody mimetics/scaffoldsAntiendomysial antibodiesPlacenta Growth Factor
The invention relates to binding partners of the placental growth factor (or placenta growth factor, PIGF), especially antibodies directed against the placental growth factor, and production and use thereof.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS PRODS
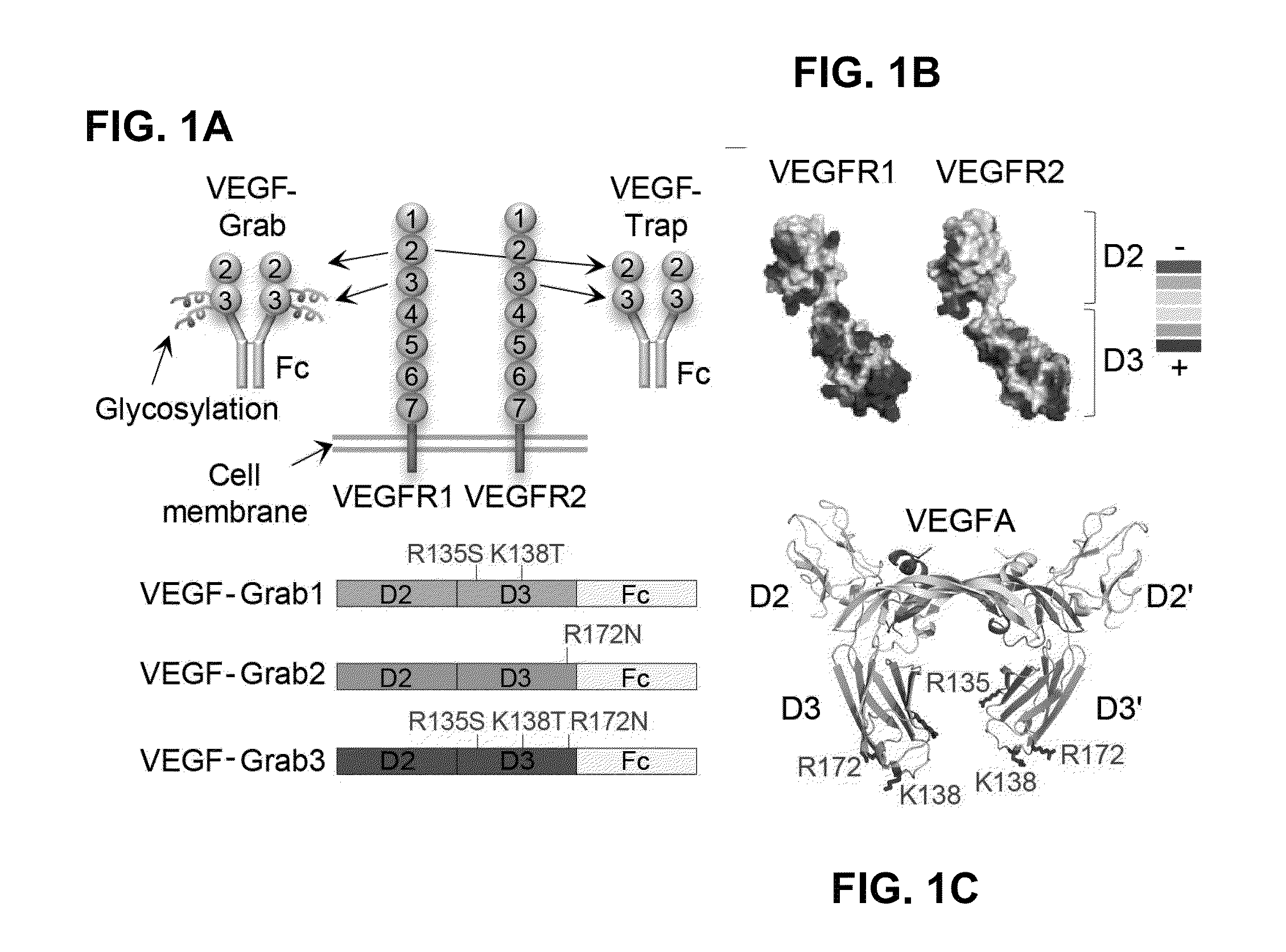
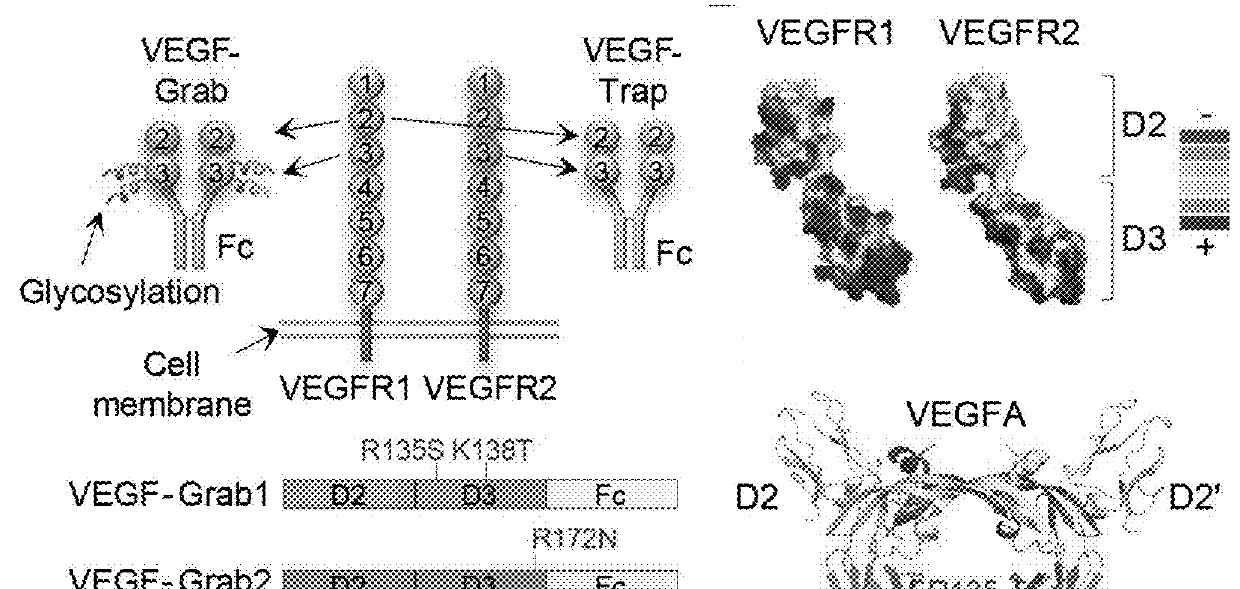
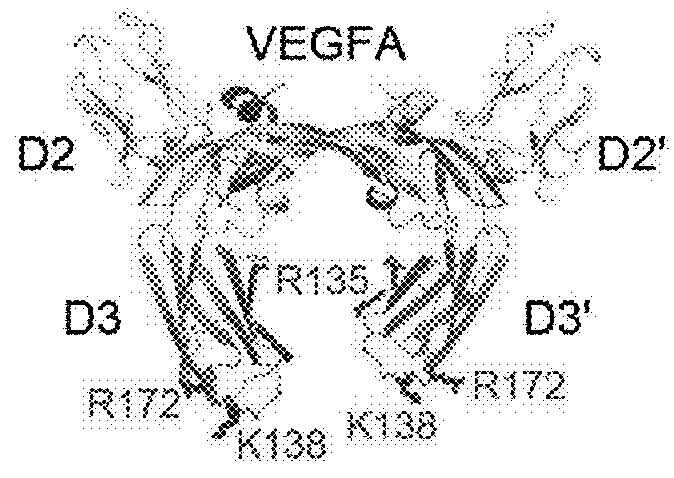
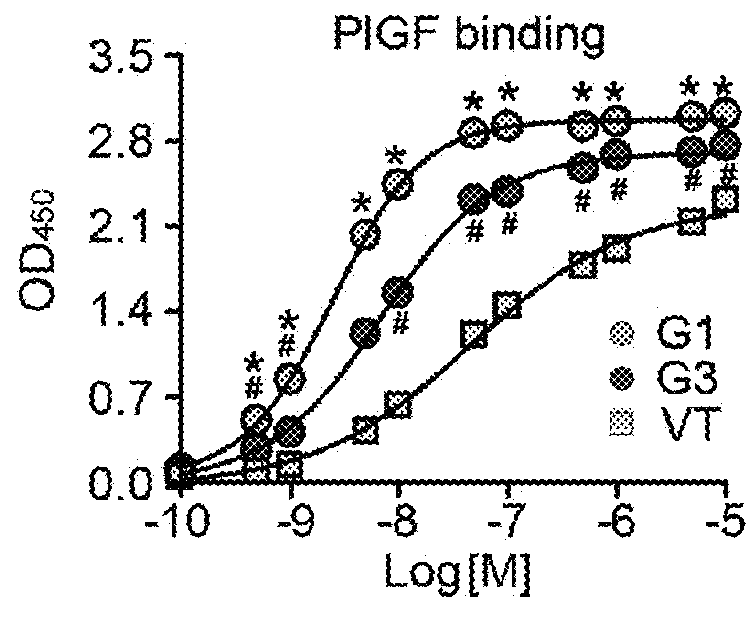
Method of treating eye disease using glycosylated VEGF decoy receptor fusion protein
ActiveUS20160024483A1Improved pharmacokinetic profileStrong and durable anti-angiogenicSenses disorderPeptide/protein ingredientsDecoy receptorsNucleotide
The present application describes an isolated nucleic acid molecule encoding a polypeptide capable of synchronously binding VEGF polypeptide and placenta growth factor (PIGF) polypeptide comprising a nucleotide sequence encoding a VEGFR1 component.
Owner:KOREA ADVANCED INST OF SCI & TECH +1
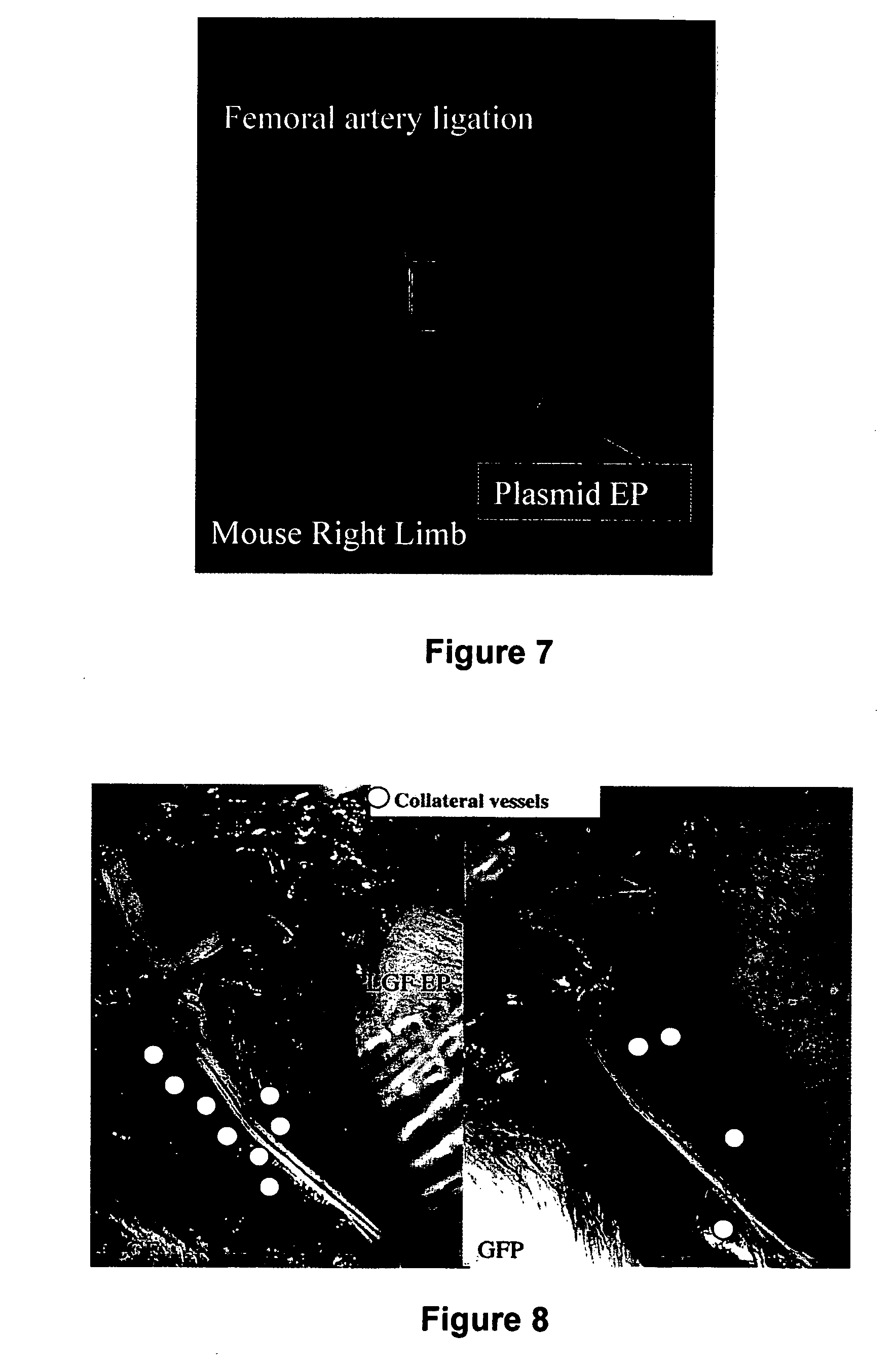
Use of placental growth factor for preventing or treating ischemic diseases or stroke
InactiveUS20070027100A1Increase perfusionReducing and suppressing infarct expansionBiocidePeptide/protein ingredientsPlacenta Growth FactorCardiac muscle
The present invention relates to prevention and treatment of strokes and ischemic diseases and to post-ischemic therapeutic treatment. The invention furthermore relates to the use of a growth factor or nucleic acids ensuring increased expression of a growth factor for treating, more particularly restoring the function of ischemic tissue, in particular muscles such as myocardium and skeletal muscles.
Owner:LIFE SCI RES PARTNERS VZW +1
Natriuretic peptides and placenta growth factor levels for risk stratification
The present invention relates to a method for determining a risk whether an individual will suffer from a cardiovascular adverse event as a consequence of cardiac stress testing, comprising the steps of (a) measuring, preferably in vitro, the level of placenta growth factor, wherein (b) if the level of the placenta growth factor is at least increased, then the individual is at least at risk of suffering from an adverse event as a consequence of cardiac stress testing. In a further embodiment, additionally another marker is measured, particularly a natriuretic peptide, most particularly NT-proBNP. The present invention allows to stratify patients according to the environment and conditions under which cardiac stress testing should be carried out.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Method for risk reduction in glycemic control
InactiveUS20110053191A1Bioreactor/fermenter combinationsBiological substance pretreatmentsPlacenta Growth FactorGlycemic
Disclosed is a method for identifying a subject being susceptible to a therapy for intensive glycemic control, the subject suffering from diabetes and being in need for a therapy for intensive glycemic control, based on determining the amount of PLGF (placental growth factor) in a sample of the subject and comparing the thus determined amount to a reference amount. In a preferred embodiment, the method further includes determining at least one further marker selected from the group consisting of a cardiac troponin and a natriuretic peptide and comparing the determined amount(s) to a reference amount (amounts). Moreover, disclosed is a method for predicting the risk of an acute cardiovascular event in a subject who suffers from diabetes and is on intensive glycemic control. Further disclosed is a kit and a device adapted to carry out the method of the present invention.
Owner:ROCHE DIAGNOSTICS OPERATIONS
Glycosylated VEGF decoy receptor fusion protein
ActiveUS20160032259A1Potent activityLimited clinical efficacySenses disorderBacteriaDecoy receptorsNucleotide
The present application describes an isolated nucleic acid molecule encoding a polypeptide capable of synchronously binding VEGF polypeptide and placenta growth factor (PIGF) polypeptide comprising a nucleotide sequence encoding a VEGFR1 component.
Owner:KOREA ADVANCED INST OF SCI & TECH
Essential oil handmade soap with effects of cooling, moisturizing and cleaning skin
InactiveCN106398926AIncrease elasticityImprove defenseSurface-active detergent compositionsSurface-active non-soap compounds and soap mixture detergentsWrinkle skinCocoyl glutamate
The invention discloses essential oil handmade soap with effects of cooling, moisturizing and cleaning skin. The essential oil handmade soap comprises, by weight, 35-45 parts of sodium cocoyl glutamate, 10-15 parts of flower and plant essential oil, 5-8 parts of tea polyphenol, 3-6 parts of lycium barbarum polysaccharide, 1-3 parts of lotus leaf extract, 1-3 parts of radix scrophulariae extract, 12-15 parts of trehalose, 7-10 parts of glutathione, 3-5 parts of vitamins, 30-35 parts of grape seed oil, 15-20 parts of palm kernel oil, 7-10 parts of castor oil, 1-2 parts of olive oil, 1.5-2.5 parts of sodium hydroxide, 5-8 parts of phosphatidylcholine bilayer, 1-2 parts of superoxide dismutase, 0.5-1 part of a placenta growth factor, 2-3 parts of royal jelly, 1-2 parts of 1, 2-propylene glycol, 2-3 parts of hydroxyethyl urea, 1-2 parts of carbomer and 2-3 parts of sodium lactate. The essential oil handmade soap has effects of deep cleaning skin, softening skin, reducing skin pruritus, balancing skin pH, supplying water, fading wrinkles, promoting shrinkage of enlarged pores of skin and keeping water-oil balance.
Owner:跨越生物科技(滁州)有限公司
Vascular endothelial growth factor fusion protein
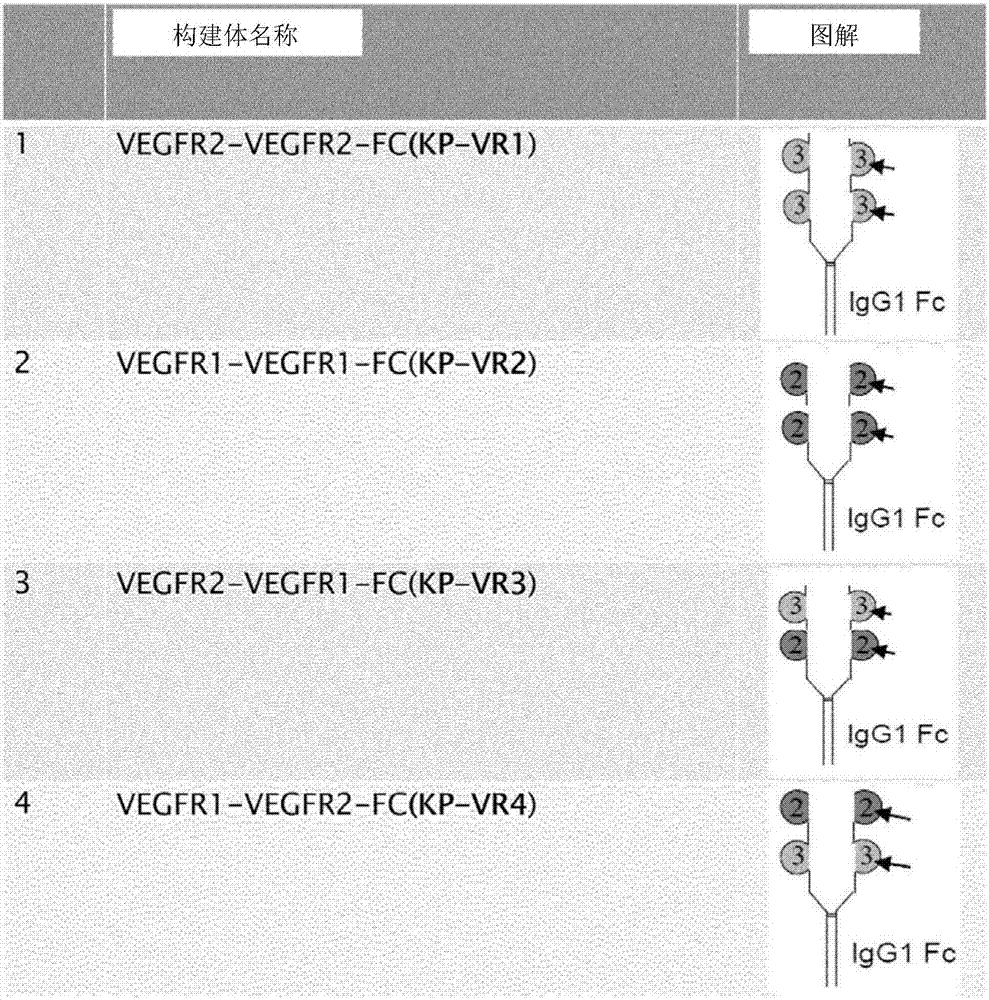
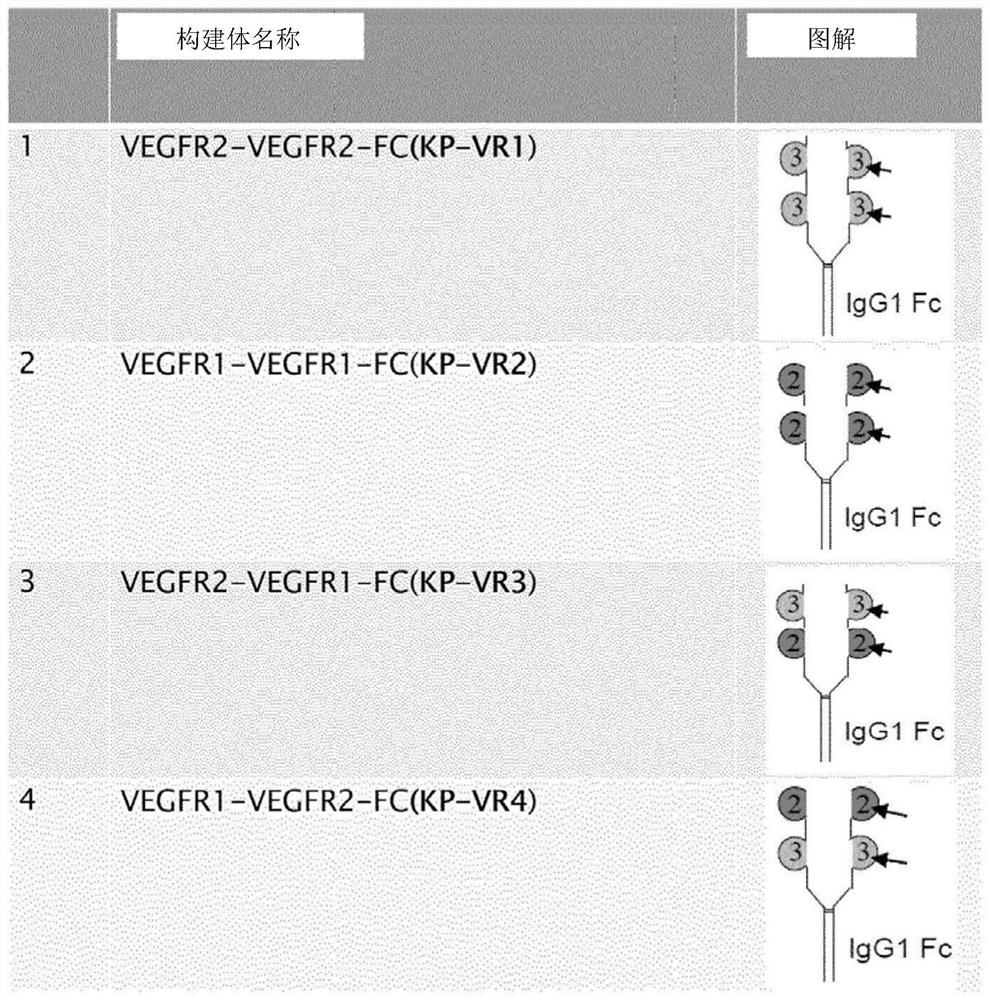
ActiveCN107312093AGrowth inhibitionPrevent proliferationSenses disorderPeptide/protein ingredientsDisulfide bondingCell invasion
The present invention relates to a vascular endothelial growth factor fusion protein. The present invention relates to a fusion protein binding to a vascular endothelial growth factor (VEGFR) and / or a placental growth factor (PLGF). The fusion protein of the present invention comprises (a) a Fc domain of IgG1, wherein two heavy chains are linked by disulfide bond, and (b) four immunoglobulin domain2s of the VEGFR1, wherein two immunoglobulin domain2s are sequentially fused to each heavy chain of the Fc domain of (a). The present fusion protein has excellent activities of inhibiting cell migration and cell invasion, and has highly enhanced growth inhibition effects to various carcinomas and fibroblasts. Therefore, the fusion protein of the present invention can be used in the preparation of an agent for treating cancers or ocular diseases.
Owner:KOREA PRIME PHARM
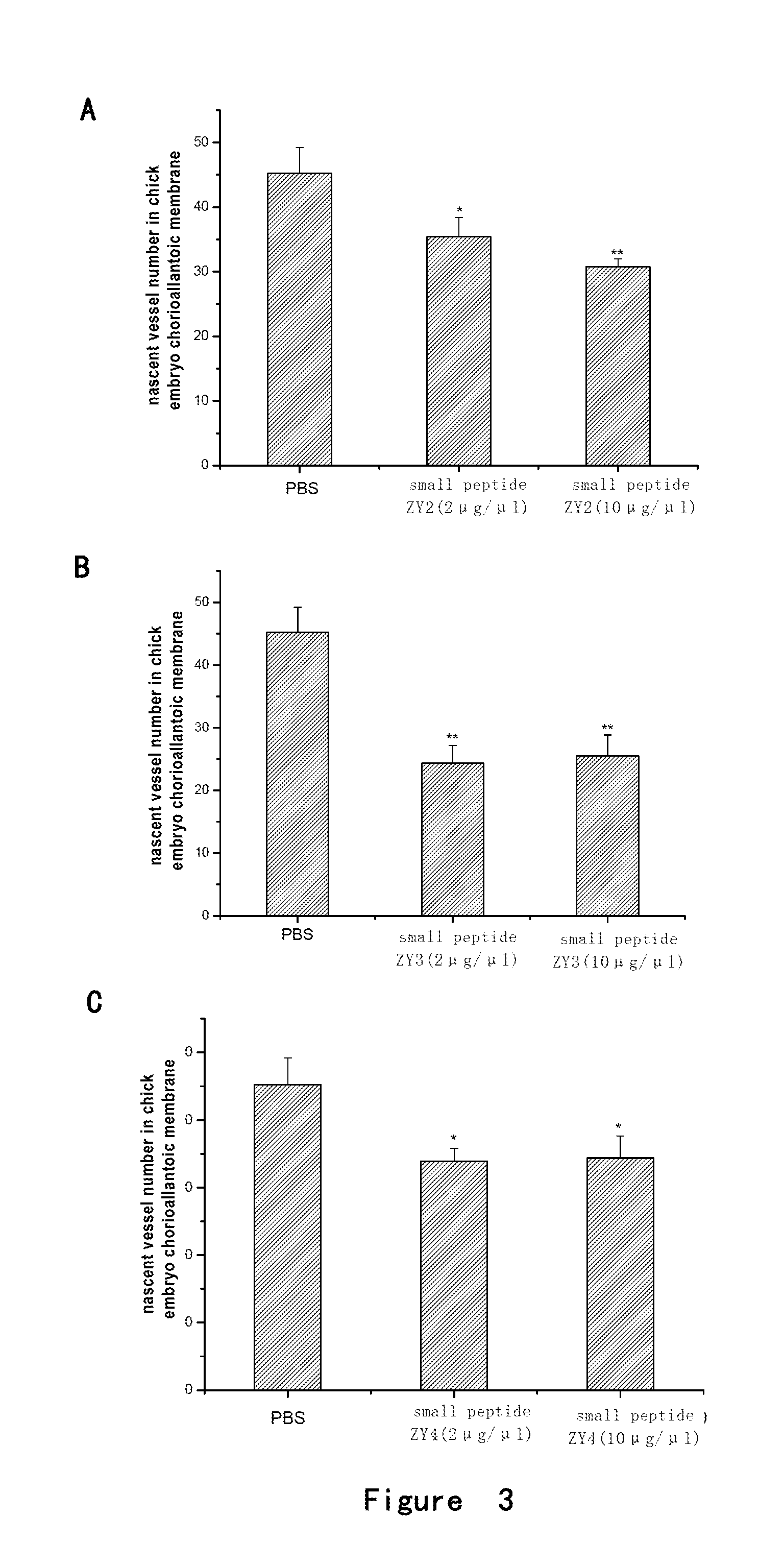
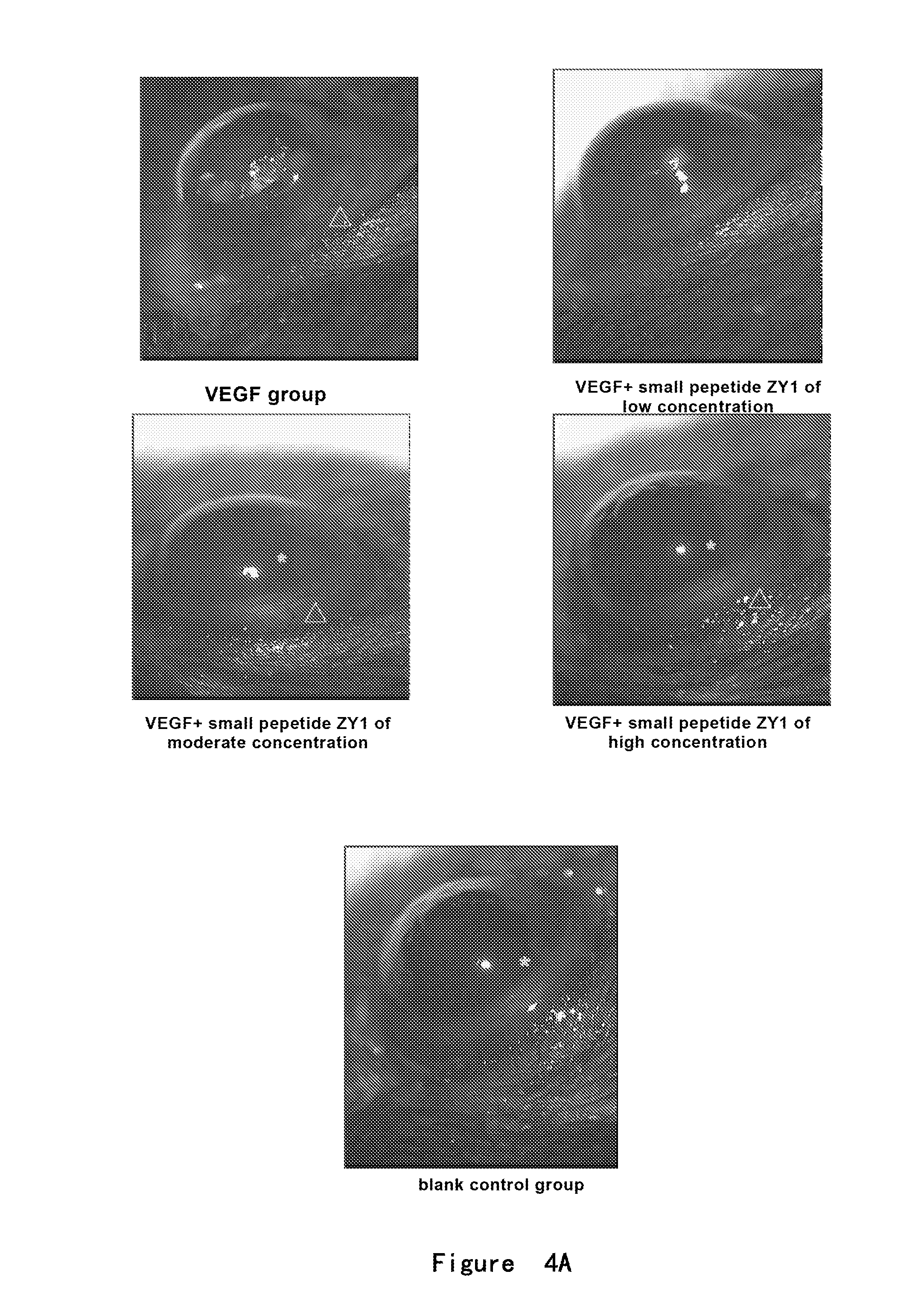
Angiogenesis-inhibiting peptide and application thereof
InactiveUS20130172258A1Inhibit angiogenesisInhibiting endothelial cell proliferationSenses disorderPeptide/protein ingredientsPlacenta Growth FactorAngiogenesis growth factor
Provided is a polypeptide having angiogenesis inhibiting activity. The polypeptide is derived from Placenta Growth Factor-1. Also provided are a derivative polypeptide of the polypeptide, a preparation method for polypeptide, and a pharmaceutical composition containing the polypeptide.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
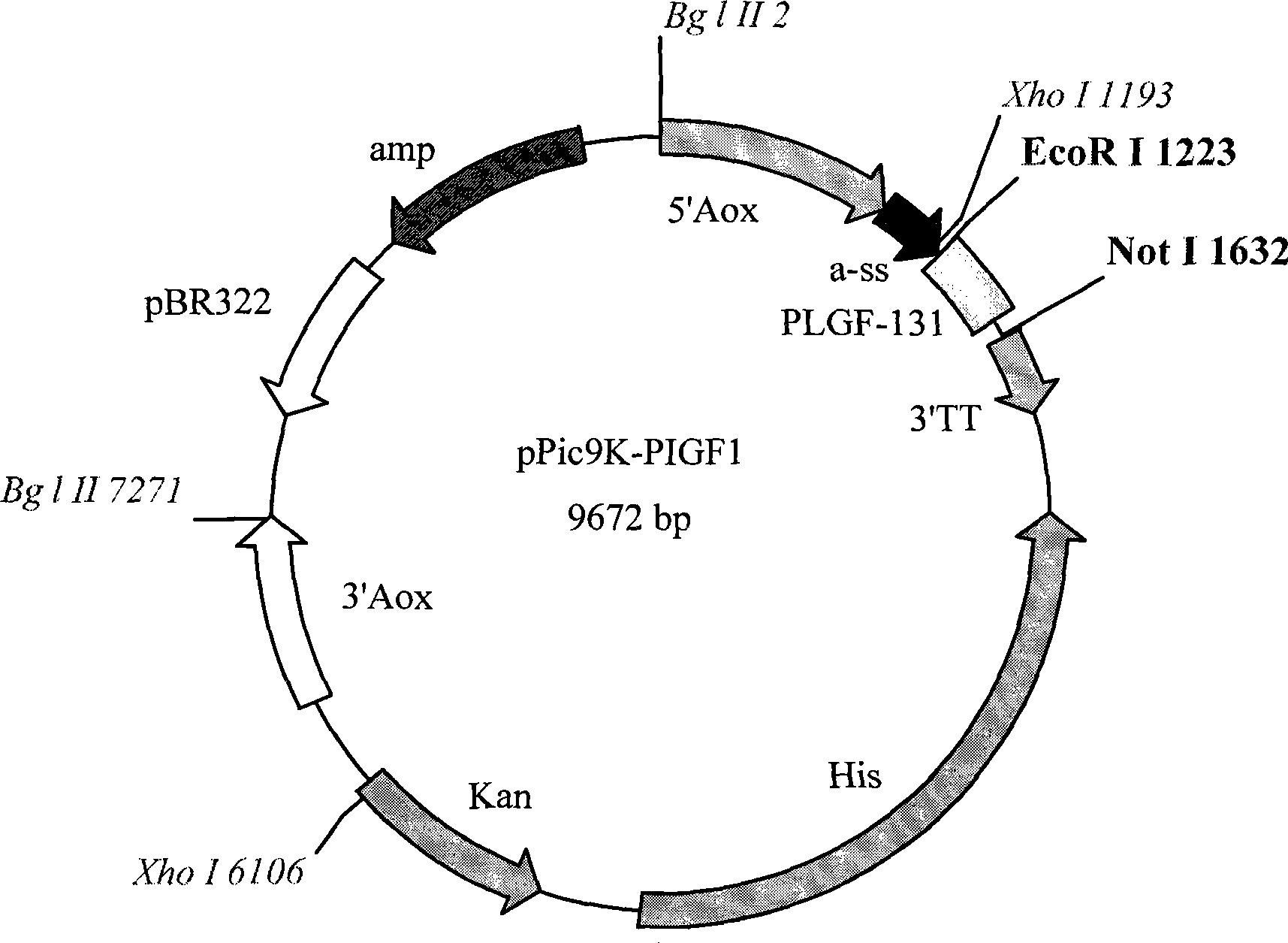
Process for preparing recombined placental growth factor
InactiveCN1620465AIncrease productionHigh specific activityPeptide preparation methodsAngiogeninInclusion bodiesIon exchange
Process for extracting and purifying the recombinant Placental Growth Factor (PLGF) expressed in inducible prokaryotic expression systems comprising the following steps: I) fermentation of the bacterial cells, II) extraction and purification of the inclusion bodies, III) renaturation of the expressed protein, IV) ion-exchange chromatography, V) reverse-phase chromatography.
Owner:GEYMONAT
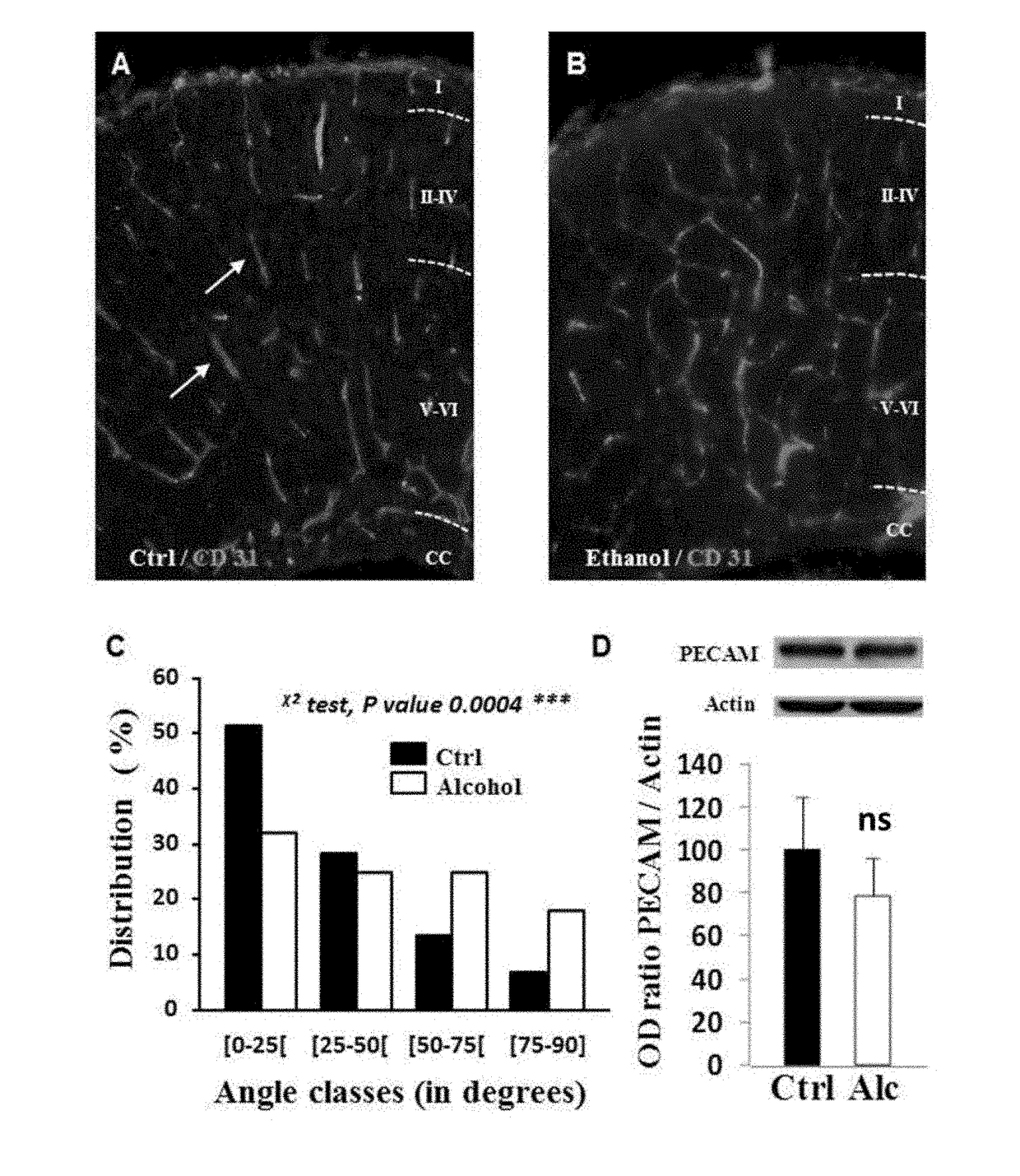
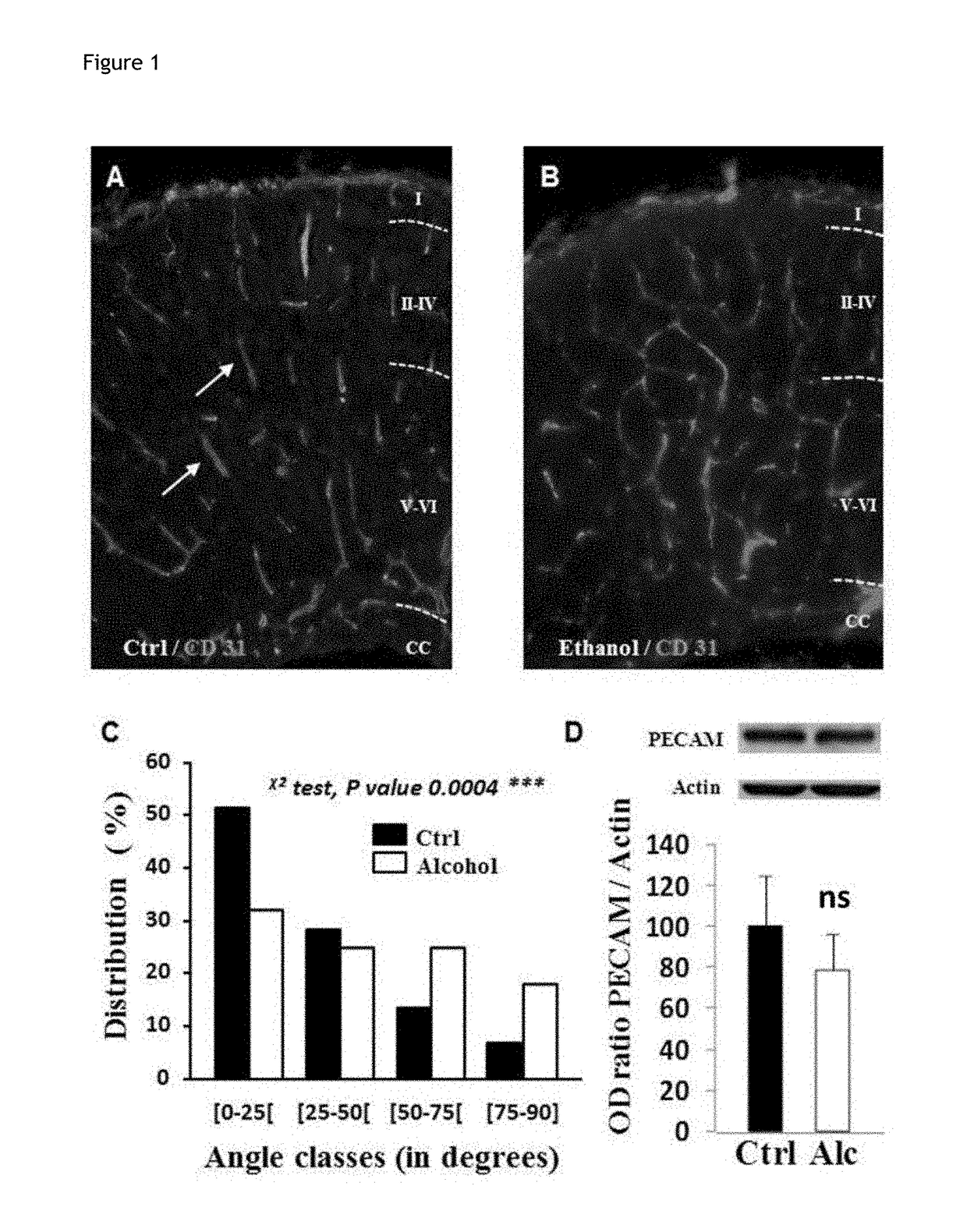
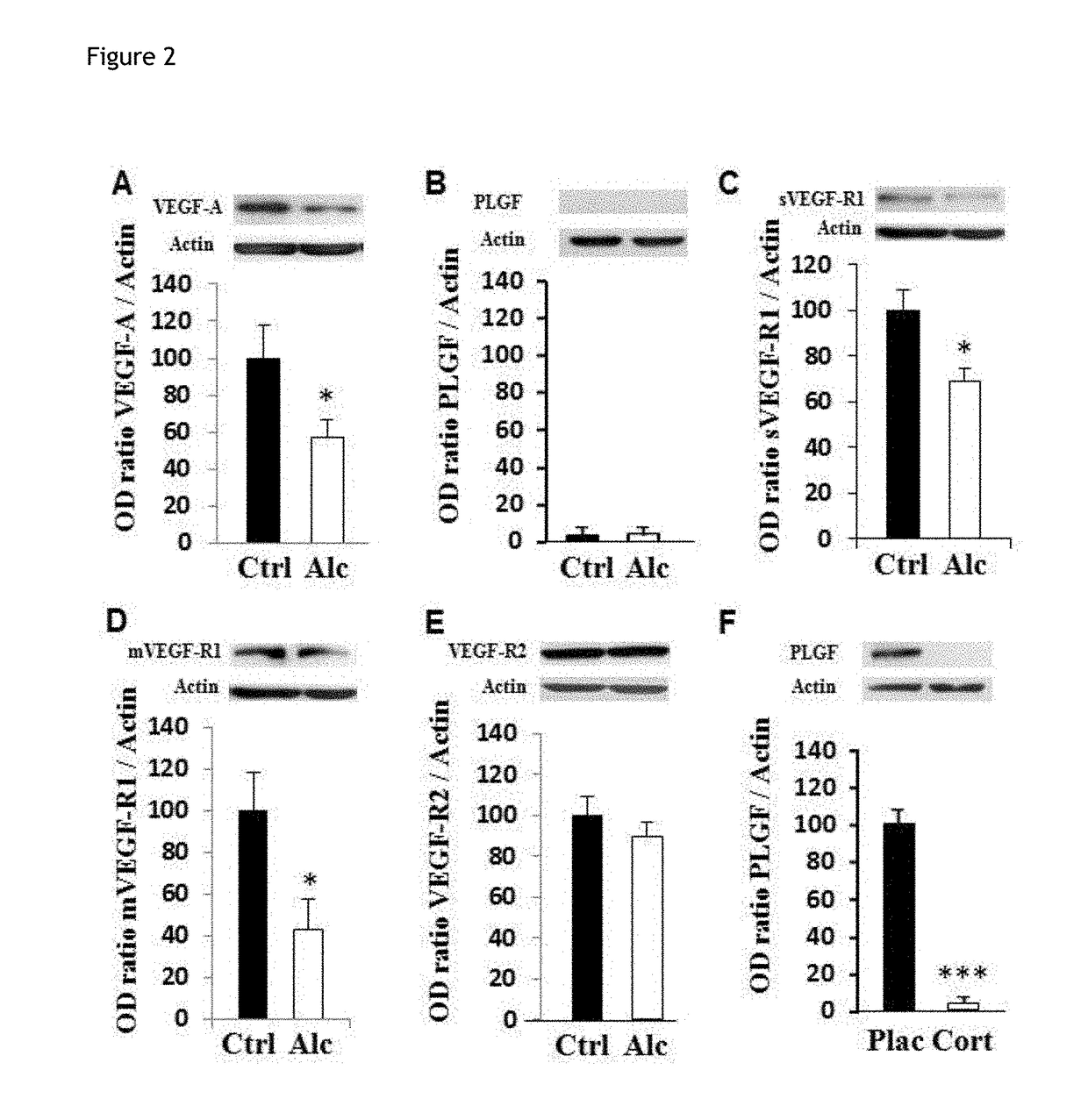
Method for the diagnosis of disorders caused by fetal alcohol syndrome
ActiveUS20180195124A1Alleviate the lackGuaranteed monitoring effectMicrobiological testing/measurementDisease diagnosisDiseasePlacenta Growth Factor
The present invention provides a method for the diagnosis of disorders caused by foetal alcohol syndrome, said method comprising the assaying of PLGF (placental growth factor).
Owner:CENT HOSPITALER UNIV DE ROUEN +2
Posterior ocular fibrosis inhibition by antagonizing placental growth factor
InactiveCN108779173ASenses disorderImmunoglobulins against growth factorsPlacenta Growth FactorFibrosis
The invention is situated in the field of ocular therapies. In particular it refers to antagonists of placental growth factor for interfering with posterior ocular fibrosis.
Owner:OXURION NV
Polypeptides inhibiting neovascularization and uses thereof
InactiveUS20150111827A1Safe for inhibit angiogenesisSafe and effectiveSenses disorderPeptide/protein ingredientsAngiogenesis growth factorPlacenta Growth Factor
Provided is a polypeptide having angiogenesis inhibiting activity. The polypeptide is derived from Placenta Growth Factor-1. Also provided are a derivative polypeptide of the polypeptide, a preparation method for polypeptide, and a pharmaceutical composition containing the polypeptide.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Angiogenesis-inhibiting peptide and application thereof
InactiveUS9023795B2Safe for inhibit angiogenesisSafe and effectiveSenses disorderPeptide/protein ingredientsPlacenta Growth FactorPharmaceutical Substances
Provided is a polypeptide having angiogenesis inhibiting activity. The polypeptide is derived from Placenta Growth Factor-1. Also provided are a derivative polypeptide of the polypeptide, a preparation method for polypeptide, and a pharmaceutical composition containing the polypeptide.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Novel compounds for the treatment of glaucoma or ocular hypertension
InactiveUS20110305710A1Avoid damageOrganic active ingredientsSenses disorderInsulin-like growth factorRetinal ganglion
This invention relates to compositions and methods for the treatment or prophylaxis of glaucoma and ocular hypertension, and for lowering of intraocular pressure. The invention provide VEGFR-1 agonists and combinations of vascular endothelial growth factor B (VEGF-B) with placenta growth factor (PLGF), ‘ Secreted protein acidic cysteine-rich’ (SPARC) antagonists, insulin, insulin like growth factor, their isoforms, their analoges, their gene-engineered modifications. Furthermore, the formentioned compositions and combinations are provided for coadministration with antiglaucoma agents, currently in clinical use, for the treatment or prophylaxis of glaucoma and ocular hypertension, for lowering of intraocular pressure, for increasing success rate of surgical procedures for the treatment or prophylaxis of glaucoma, and for preserving retinal ganglion cells.
Owner:ABDULRAZIK MUHAMMAD
Polypeptides inhibiting neovascularization and uses thereof
InactiveUS9266933B2Safe for inhibit angiogenesisSafe and effectiveSenses disorderPeptide/protein ingredientsPlacenta Growth FactorAngiogenesis growth factor
Provided is a polypeptide having angiogenesis inhibiting activity. The polypeptide is derived from Placenta Growth Factor-1. Also provided are a derivative polypeptide of the polypeptide, a preparation method for polypeptide, and a pharmaceutical composition containing the polypeptide.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Soluble fms-like tyrosine kinase-1 detection kit as well as preparation method and application thereof
PendingCN114252592AHigh sensitivityEasy to detectChemiluminescene/bioluminescenceDisease diagnosisTyrosinePlacenta Growth Factor
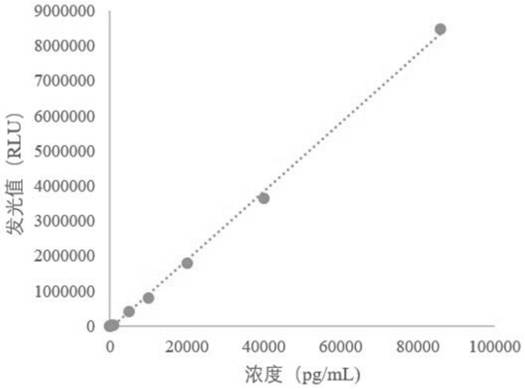
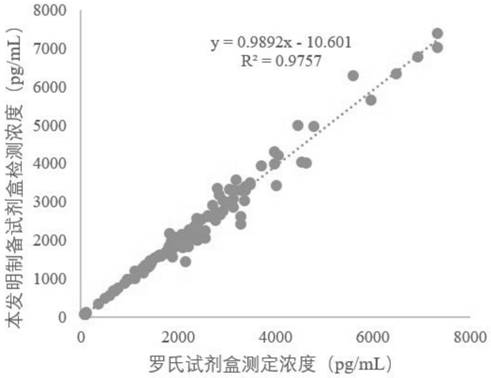
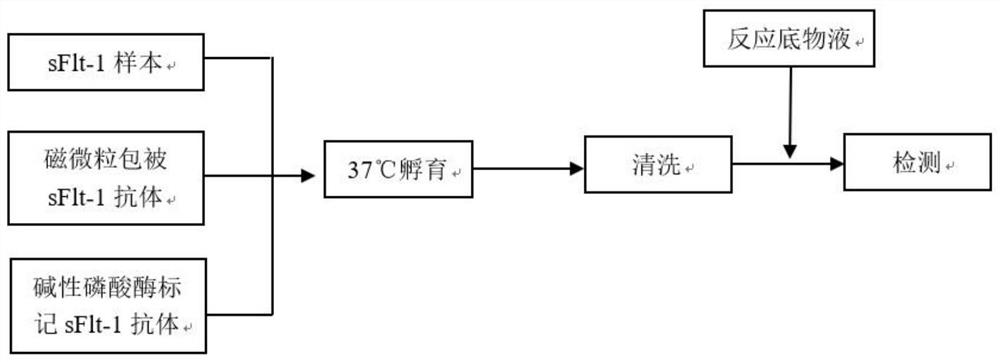
The invention relates to a soluble fms-like tyrosine kinase-1 detection kit as well as a preparation method and application thereof, the inventor detects soluble fms-like tyrosine kinase-1 by adopting a double-antibody sandwich method combined with a magnetic particle chemiluminescence technology, and finds that an immune micro-magnetic particle preparation method optimized by the inventor is adopted. A soluble fms-like tyrosine kinase-1 chemiluminescence immunoassay kit is formed by the prepared placenta growth factor capture antibody magnetic particles and an alkaline phosphatase labeled placenta growth factor detection antibody which is further prepared by the inventor through cross-linking agents DTBP and DTT and a sealing agent MMTS in a specific proportion. According to the present invention, the detection can be automatically completed by using the full-automatic chemiluminescence immunoassay analyzer as the detection tool, the detection performance is significantly improved, the detection sensitivity achieves 0.97 pg / mL, and the detection linear range is wide and can achieve 10-85000 pg / mL. Therefore, the method has a wide detection application prospect.
Owner:GUANGZHOU WONDFO BIOTECH
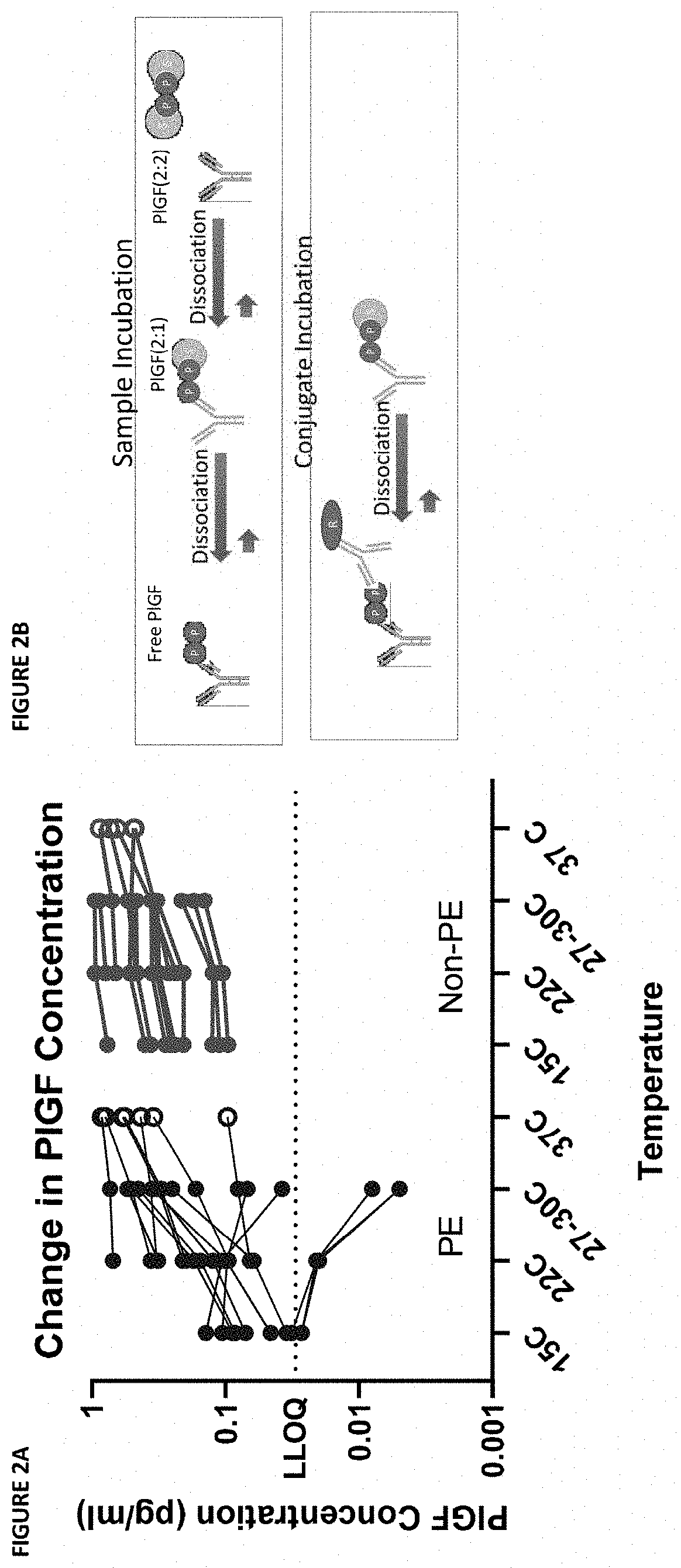
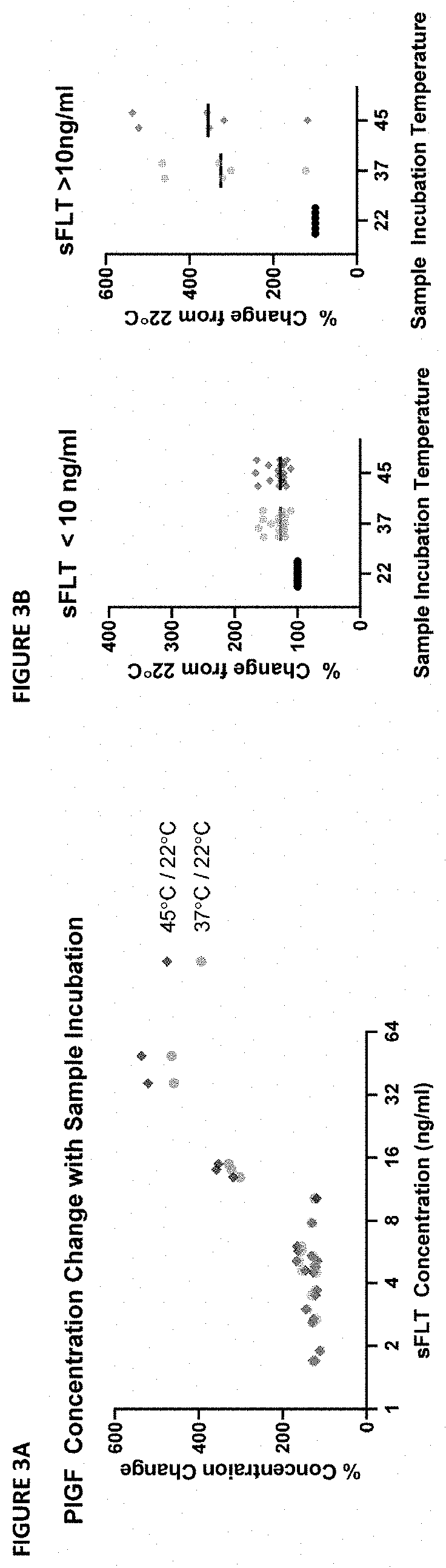
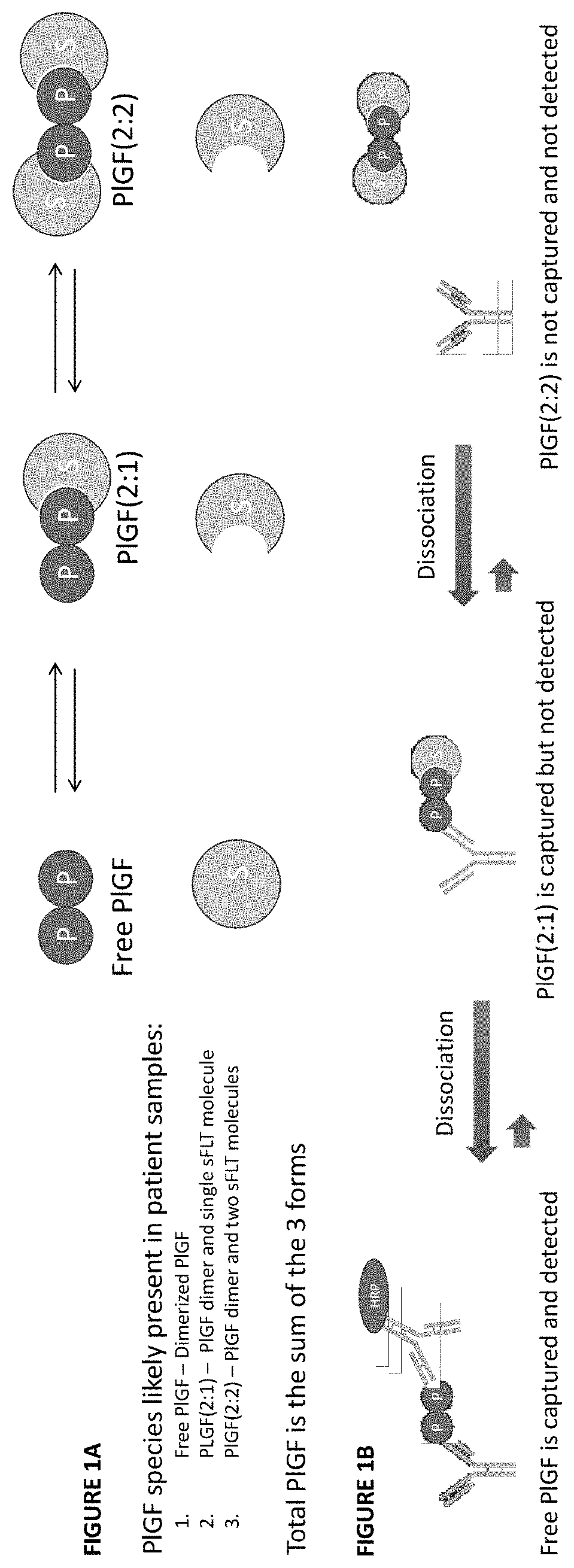
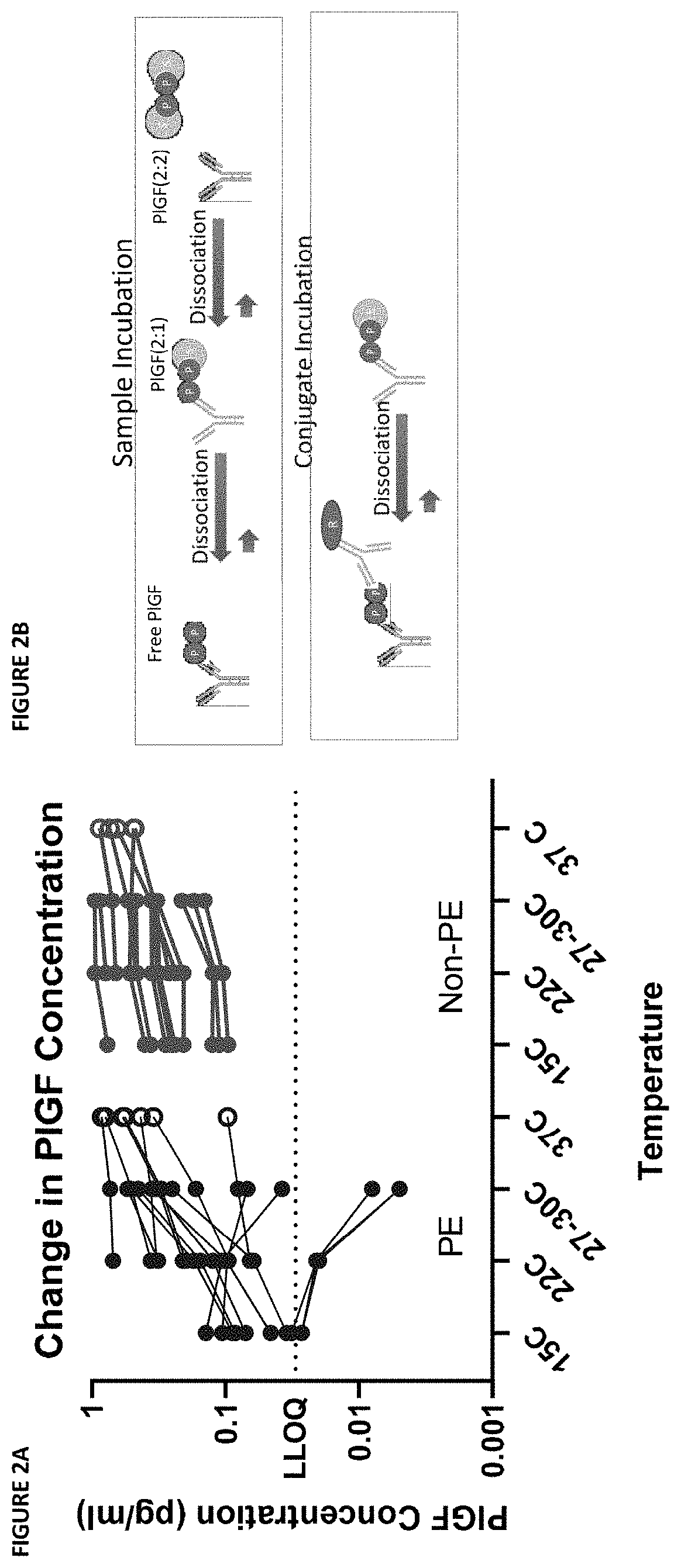
Assessment of preeclampsia using assays for free and dissociated placental growth factor
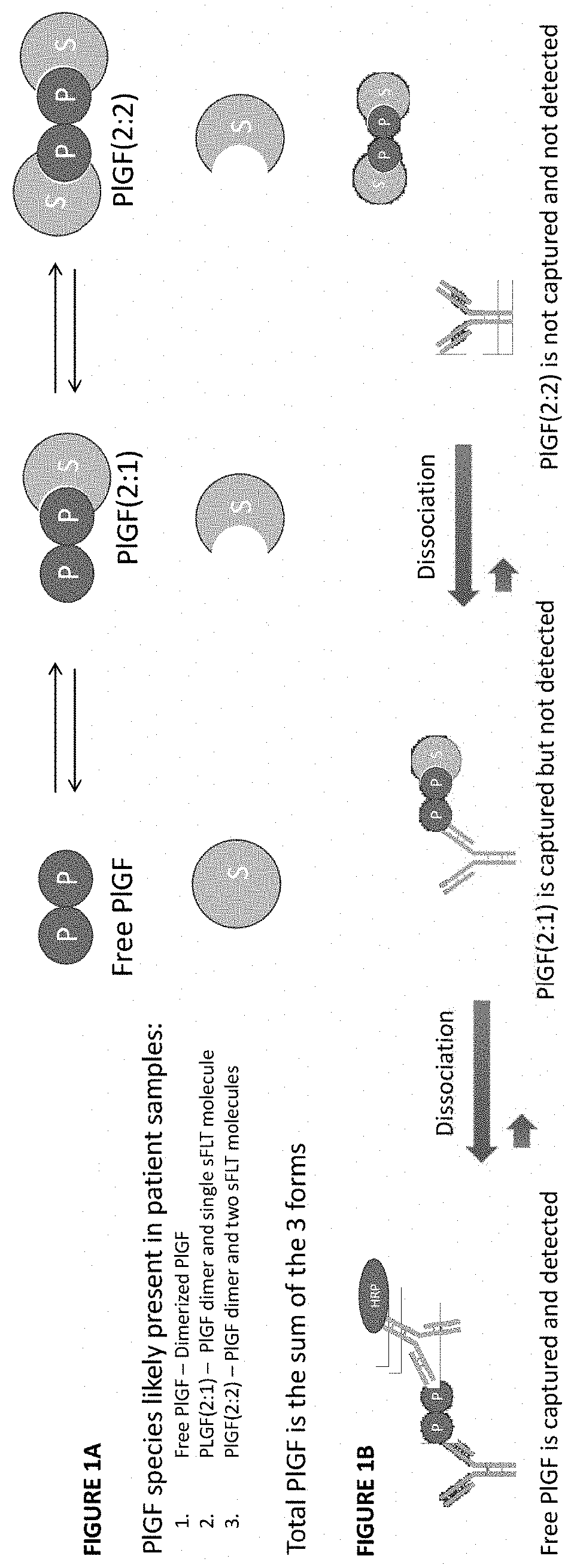
ActiveUS20210172938A1Avoiding unnecessary treatmentAvoid treatmentDisease diagnosisBiological testingObstetricsAssay
Described herein are methods, compositions, kits, and systems for detecting free and bound PlGF, and using detection of such species to distinguish between pregnant women with or without preeclampsia or related conditions.
Owner:BIORA THERAPEUTICS INC
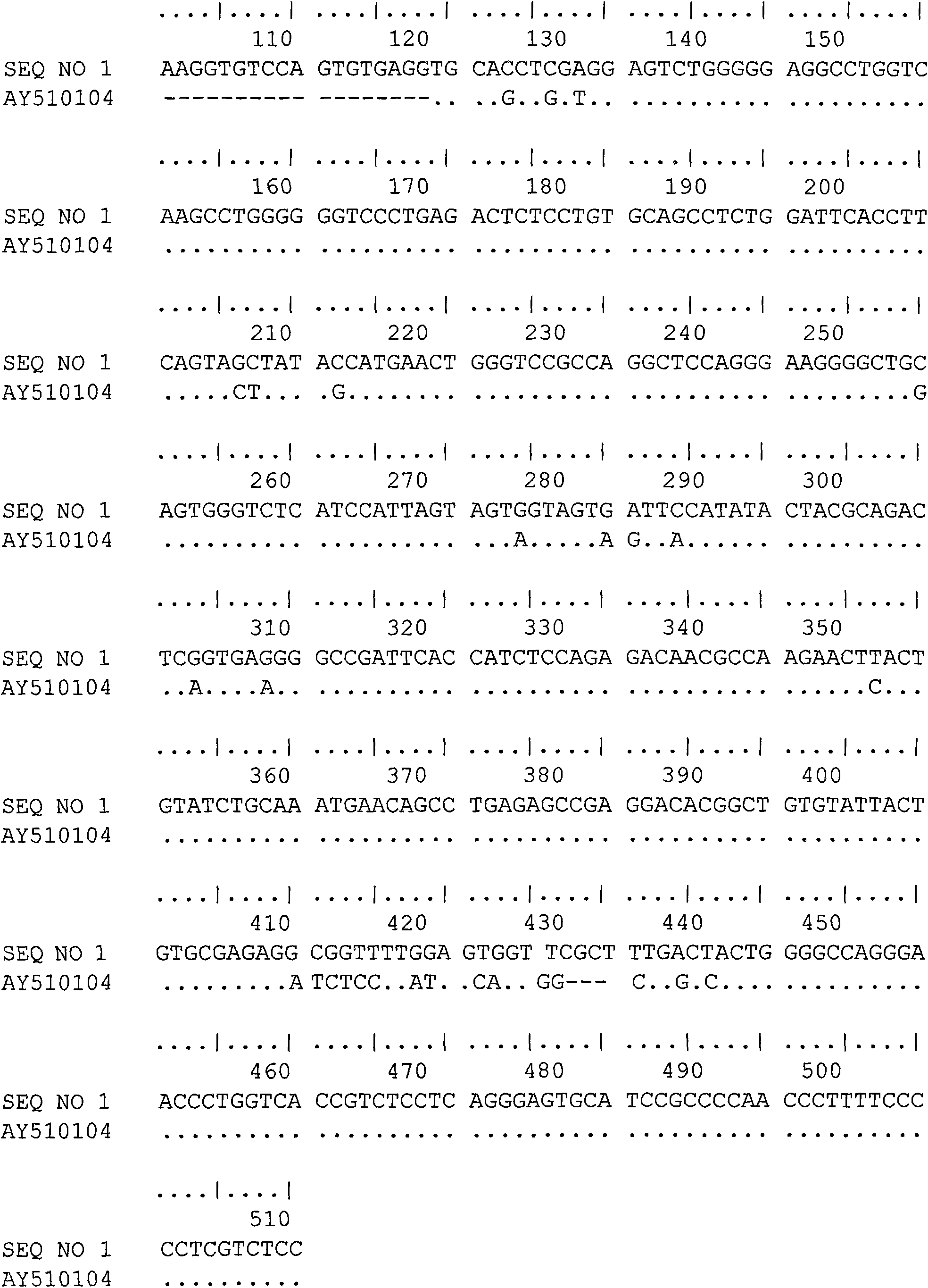
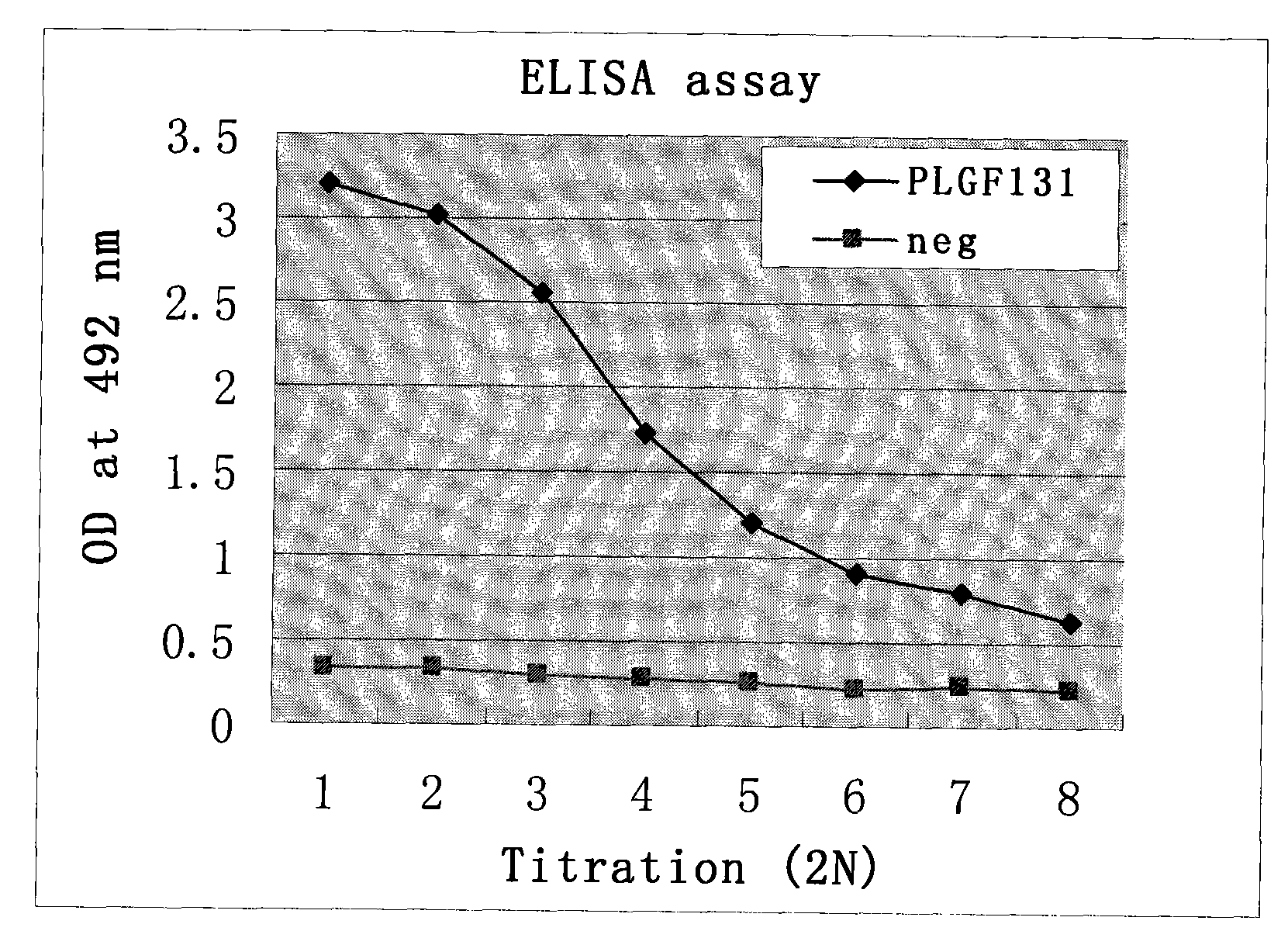
Single chain antibody of human anti-placenta growth factor
InactiveCN101643508AHigh affinityImmunoglobulins against growth factorsPeptide preparation methodsAntigenCDNA library
The invention relates to a single chain antibody of human anti-placenta growth factor, which is encoded by a heavy-chain gene with an SEQ NO.1 sequence and light-chain gene with an SEQ NO.2 sequence.The invention also provides a preparation method thereof, comprising the following steps in sequence: amplifying human total RNA heavy-chain variable region, light-chain variable region and constant region gene of the whole set by a PCR technique, splicing and constructing an overall-length single-chain antibody cDNA library; expressing the cDNA library by an externally-coupled transcription / translation system to obtain an antibody-ribosome-mRNA compound; performing affiliation selection and clution on the compound by a magnetic bead coated by specific antigen; amplifying selected destinationantibody gene by the PCR technique; and expressing the amplified antibody gene using the externally-coupled transcription / translation system repeatedly to obtain the single-chain antibody. The antibody is adapted to prepare medicament for treating ovarian cancer.
Owner:SHANGHAI TENTH PEOPLES HOSPITAL
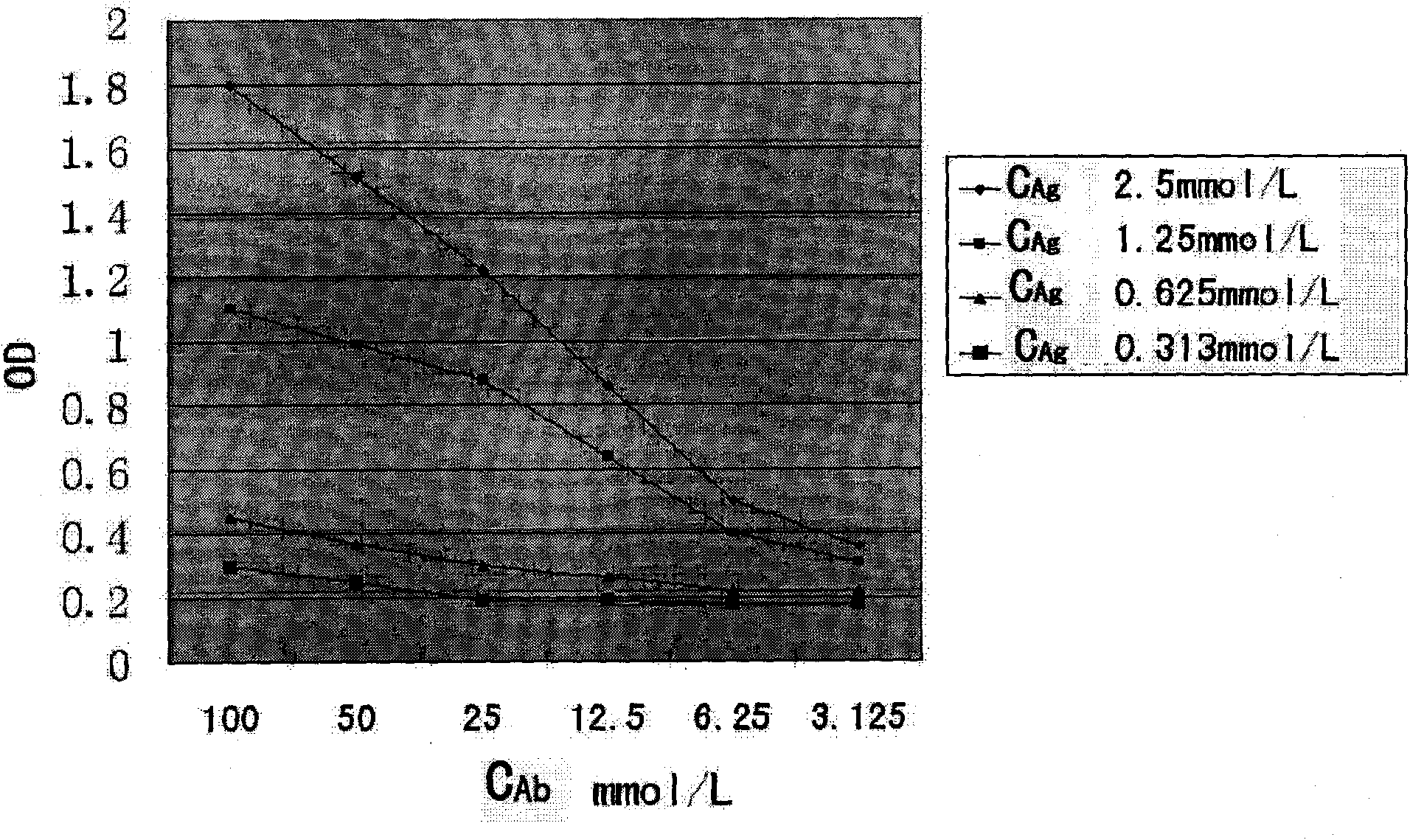
Placenta growth factor quality control product and preparation method thereof
PendingCN113834942AReduce the differenceEnsure stabilityBiological material analysisBiological testingAntigenFluorescence immunoassay
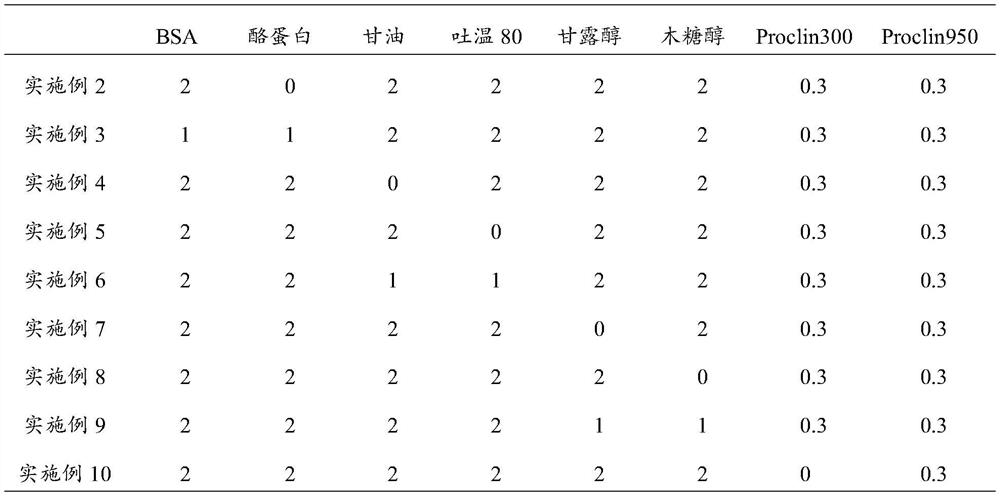
The invention provides a placenta growth factor quality control product and a preparation method thereof. The placenta growth factor quality control product comprises a placenta growth factor antigen, newborn calf serum, BSA and / or casein, glycerin and / or Tween 80, mannitol and / or xylitol and a preservative. The placenta growth factor quality control product disclosed by the invention is simple in preparation method, high in accuracy, high in precision and good in stability, can be applied to a fluorescence immunoassay platform of a POCT system, and is suitable for placenta growth factor detection kits clinically common on the platform. A quality control substance is provided for placenta growth factor kit evaluation and indoor and interventricular quality evaluation in clinical laboratories.
Owner:HEBEI TEWENTE BIOTECH DEV CO LTD
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a one or more assays configured to detect a kidney injury marker selected from the group consisting of Heat shock protein beta-1, WAP four-disulfide core domain protein 2, Choriogonadotropin subunit beta, Placenta growth factor, and Mitochondrial 60 kDa heat shock protein as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Preparation method of chemical luminescence kit for placenta growth factors
InactiveCN110308287AImprove capture efficiencyUniform particle sizeChemiluminescene/bioluminescenceBiological testingPlacenta Growth FactorPolystyrene
The invention discloses a preparation method of a chemical luminescence kit for placenta growth factors. The preparation method comprises the following steps of preparing streptavidin magnetic particles, and preparing a streptavidin magnetic particle suspension liquid, preparing a placenta growth factor marked by biotin, preparing a placenta growth factor antibody marked by a chemical luminescencesubstance and the like; the streptavidin magnetic particles have high affinity between streptavidin and biotin, so that any biotin-labeled molecules can be bound, and the streptavidin magnetic particles can be used for immunization and molecular detection; the preparation method adopts the polystyrene magnetic particles, and has the characteristics of uniform particle size, large specific surfacearea and regular morphology, so that the target molecule can be rapidly and efficiently captured and magnetic separation is realized; and the magnetic particle chemiluminescence method provided by the invention has strong specificity, accuracy and quickness, short in detection time, accurate in detection result, high in repeatability and the like.
Owner:NINGBO AUCHEER BIOTECHNOLOGY CO LTD
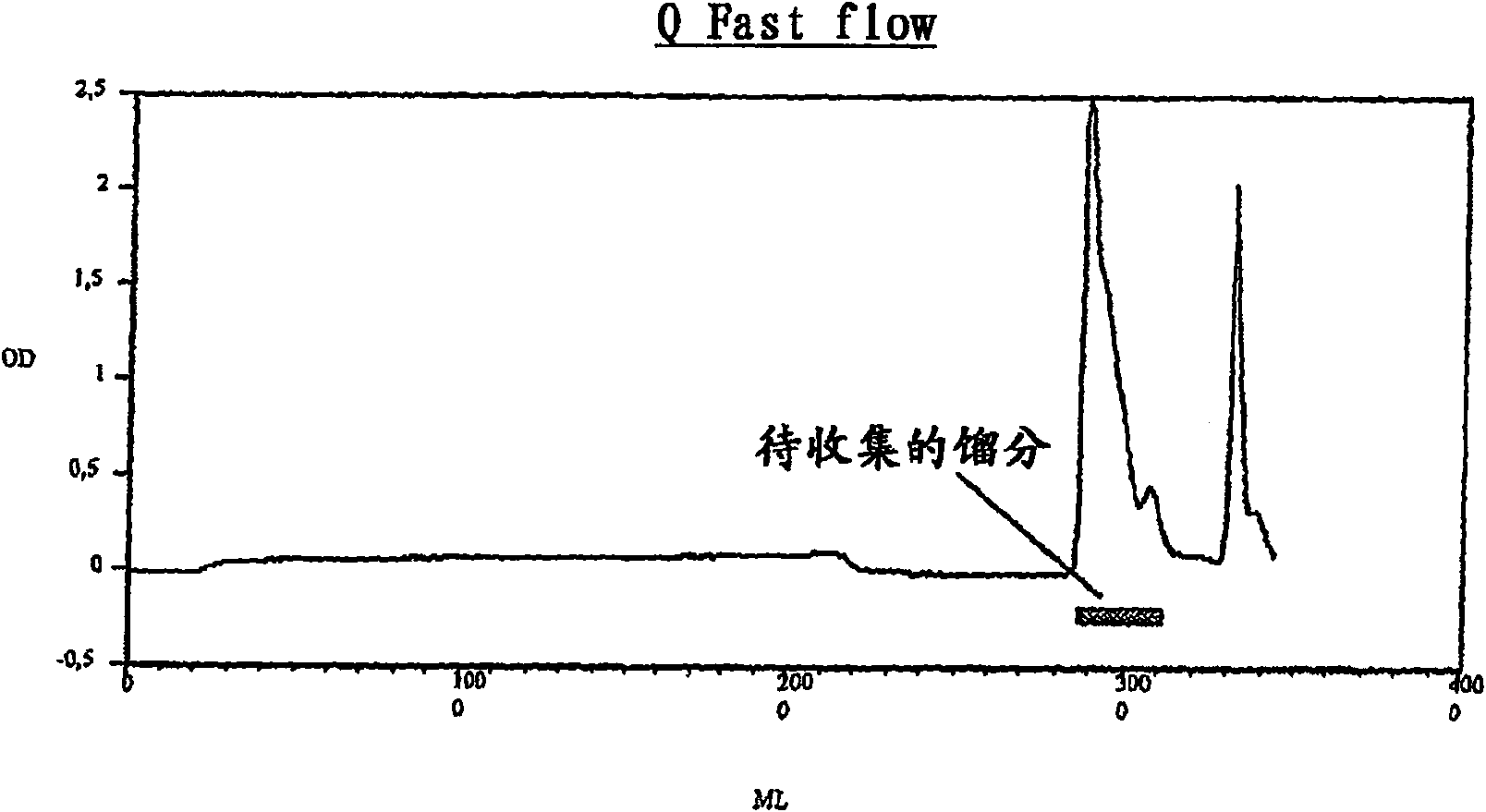
Expression production and separation purification of recombinant placenta growth factor and chemical marker thereof
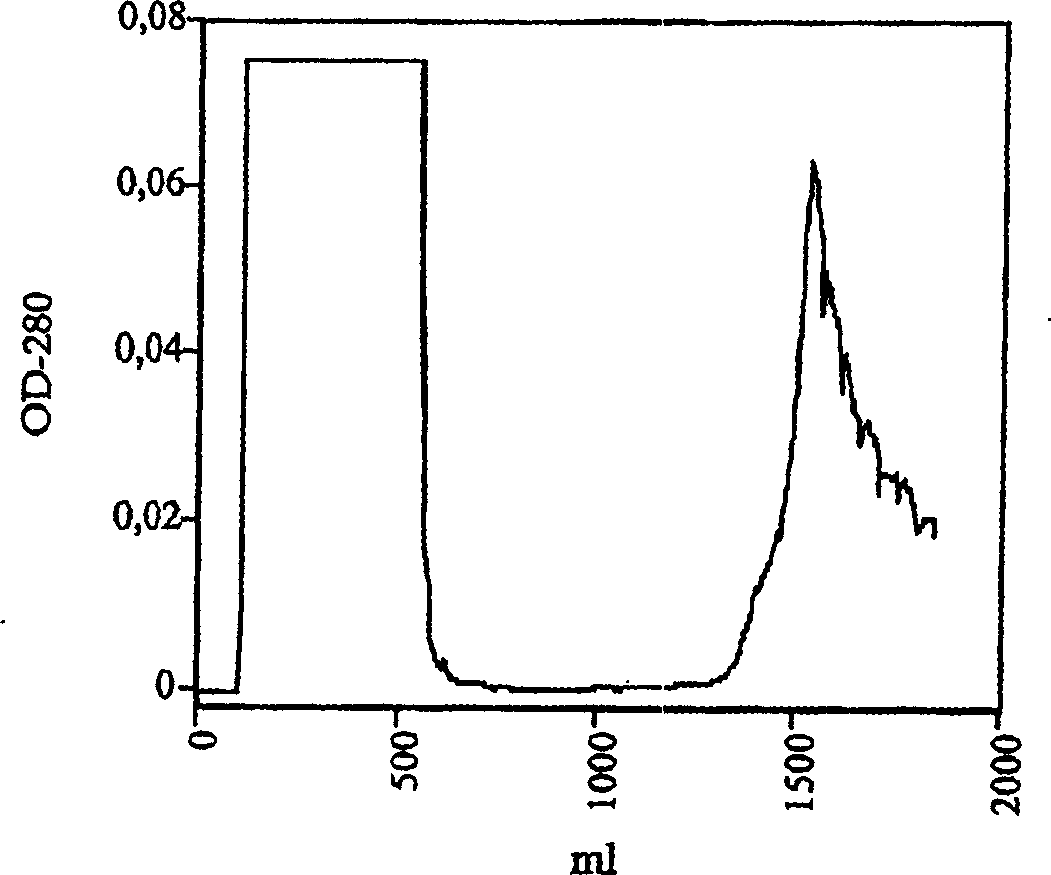
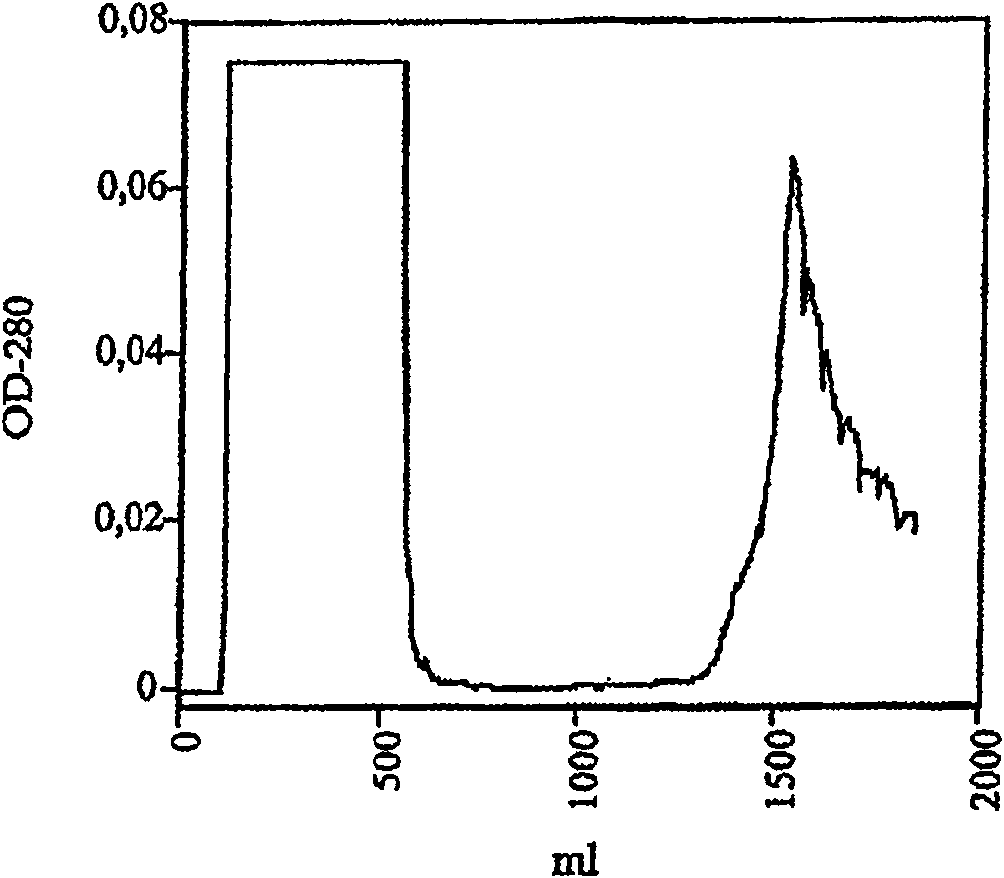
ActiveCN101497883AEasy to separatePeptide preparation methodsBiological testingVascular proliferationSeparation technology
The invention belongs to the field of biological technique, i.e. gene recombination engineering. Placental growth factors (PLGFs) play important roles in neovascularization and vascular proliferation; however, natural PLGF content in vivo is low and the natural PLGFs have short half-times, thus to extract sufficient entogenous PLGF proteins so as to satisfy the increasingly demands of experimental study and clinical practice is difficult. The invention discloses a method for efficiently expressing, producing and recombining a PLGF system in vitro by gene recombination engineering and cell culture technology and for extracting and purifying the PLGF proteins from expressed supernatant by chromatographic separating technology. The invention also discloses a nonradioactive chemical coupling labeling treatment method for the purified PLGF proteins in vitro. The PLGF proteins (including the unlabeled or the chemical coupling labeled) have wide application in the experimental study and the clinical practice.
Owner:ACROIMMUNE BIOTECH CO LTD
Assessment of preeclampsia using assays for free and dissociated placental growth factor
Described herein are methods, compositions, kits, and systems for detecting free and bound PlGF, and using detection of such species to distinguish between pregnant women with or without preeclampsia or related conditions.
Owner:BIORA THERAPEUTICS INC
Placenta growth factor in treating duchenne muscular dystrophy
ActiveUS20150368309A1Increase the amount addedImprovement in DMD symptomPeptide/protein ingredientsAntibody mimetics/scaffoldsMedicinePlacenta Growth Factor
The present invention provides, among other things, methods and compositions for treating muscular dystrophy, in particular, Duchenne muscular dystrophy (DMD). In some embodiments, a method according to the present invention includes administering to an individual who is suffering from or susceptible to DMD an effective amount of a recombinant PLGF protein such that at least one symptom or feature of DMD is reduced in intensity, severity, or frequency, or has delayed onset. The present invention also provides exemplary recombinant PLGF proteins including monomeric, dimeric and single-chain PLGF proteins.
Owner:TAKEDA PHARMA CO LTD
Vascular endothelial growth factor fusion protein
ActiveCN107312093BGrowth inhibitionPrevent proliferationSenses disorderPeptide/protein ingredientsDisulfide bondingCell invasion
The name of the present invention is vascular endothelial growth factor fusion protein. The present invention relates to fusion proteins that bind to vascular endothelial growth factor (VEGFR) and / or placental growth factor (PlGF). The fusion protein of the present invention comprises (a) the Fc domain of IgG1, wherein two heavy chains are linked by disulfide bonds, and (b) four immunoglobulin domains 2 of VEGFR1, of which two immunoglobulin domains 2 Each heavy chain fused sequentially to the Fc domain of (a). The fusion protein of the present invention has excellent activity of inhibiting cell migration and cell invasion, and has highly enhanced growth inhibitory effect on various cancers and fibroblasts. Therefore, the fusion protein of the present invention can be used to prepare medicaments for treating cancer or eye diseases.
Owner:KOREA PRIME PHARM
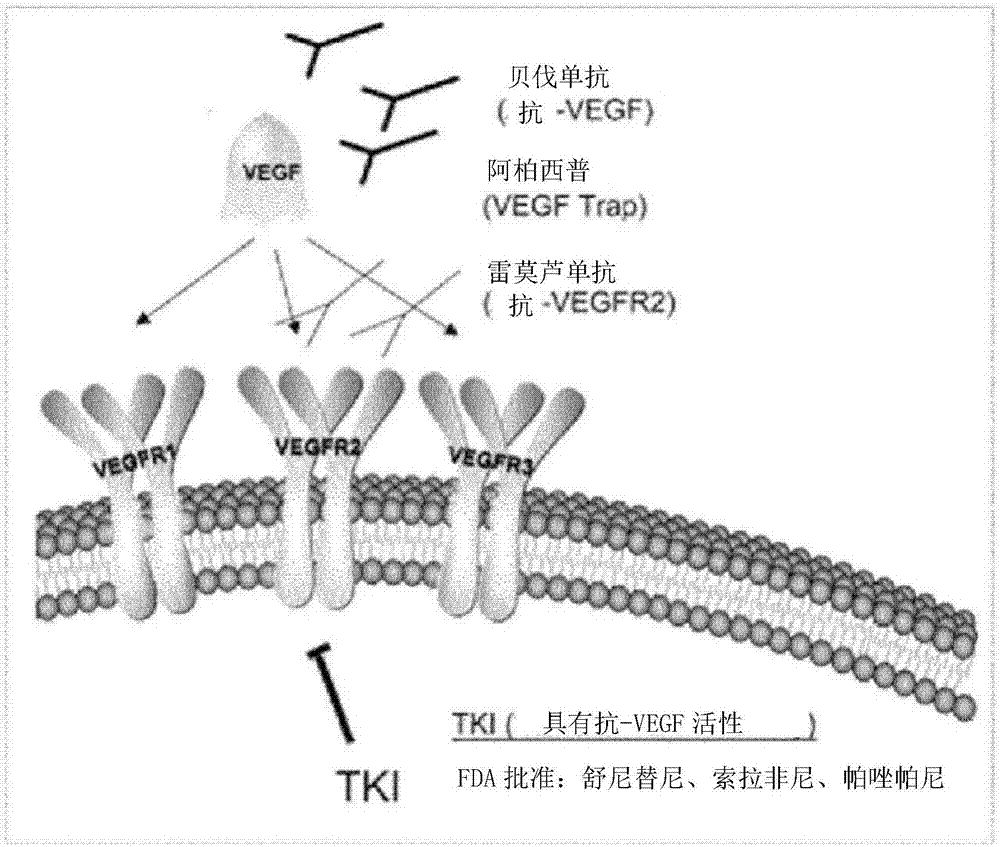
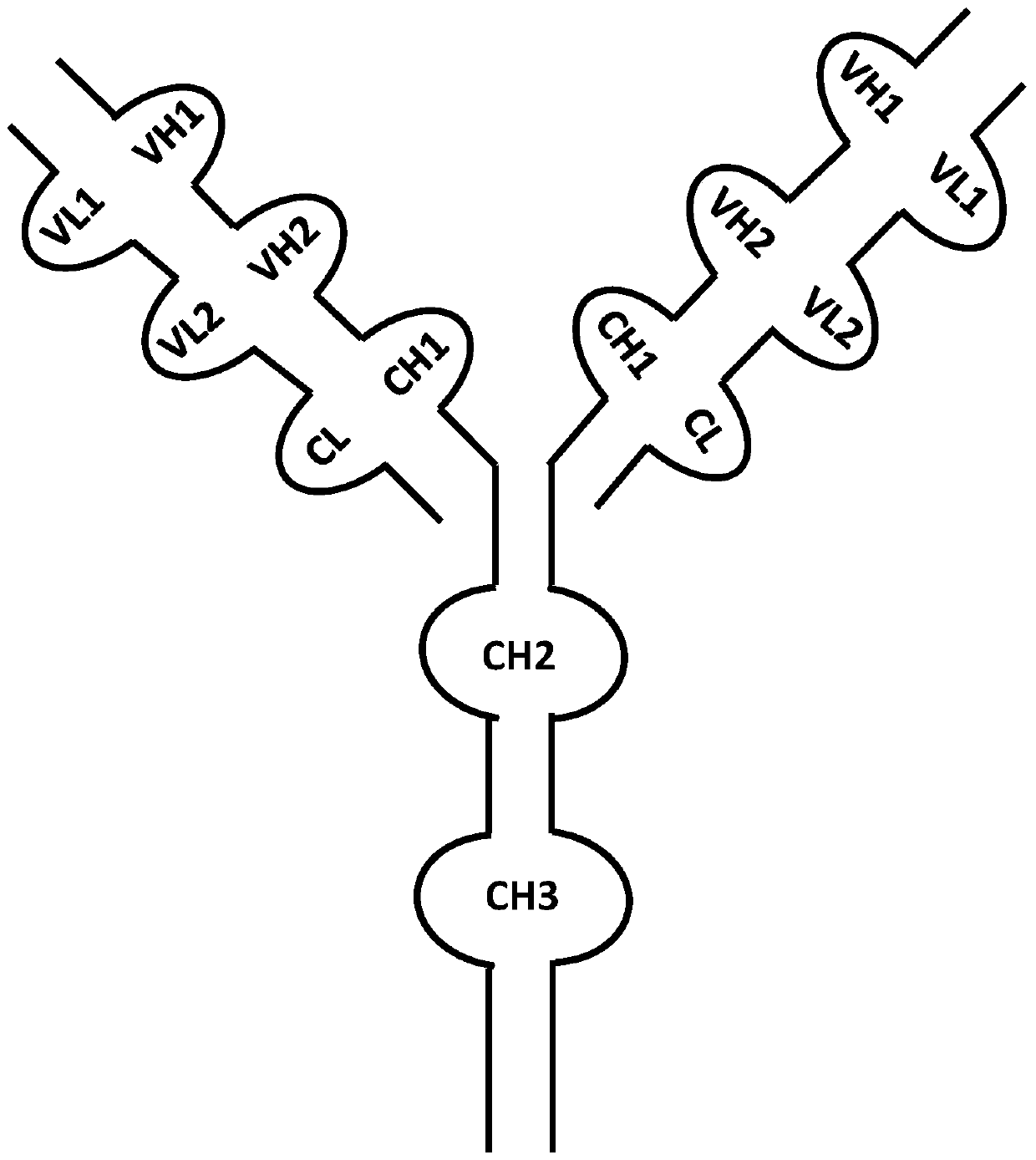
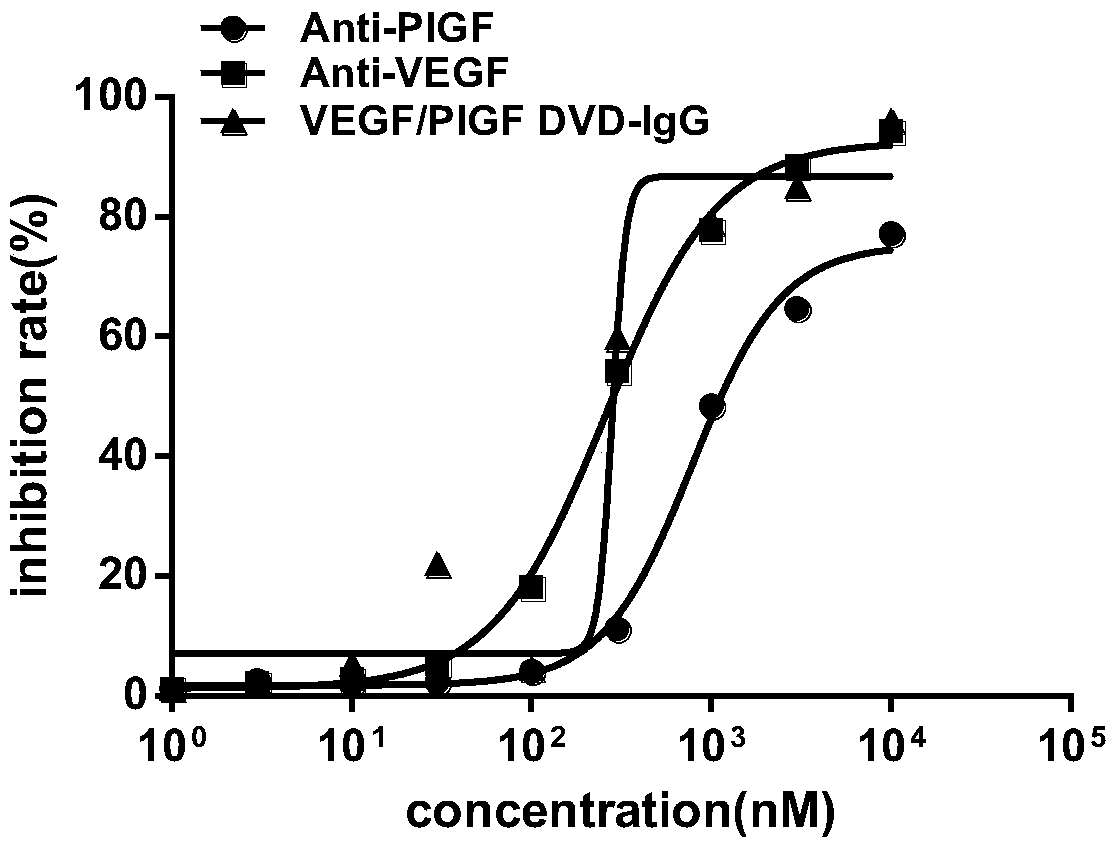
Anti-vegf/pigf bispecific antibody, its preparation method and use
ActiveCN104974259BInhibit synthesisHybrid immunoglobulinsImmunoglobulins against growth factorsAbnormal tissue growthPlacenta Growth Factor
The invention belongs to the field of biological technologies, and more specifically discloses an anti-VEGF / PIGF (vascular endothelial growth factor / placenta growth factor) bispecific antibody as well as a preparation method and an application thereof. The anti-VEGF / PIGF bispecific antibody can be combined with VEGF and PIGF, and can effectively inhibit the formation of tumor internal blood vessels, thereby inhibiting the growth of tumors more effectively.
Owner:SUNSHINE GUOJIAN PHARMA (SHANGHAI) CO LTD
Process for preparing recombined placental growth factor
InactiveCN100588661CImprove biological activityPeptide preparation methodsAngiogeninInclusion bodiesPlacenta Growth Factor
Process for extracting and purifying the recombinant Placental Growth Factor (PLGF) expressed in inducible prokaryotic expression systems comprising the following steps: I) fermentation of the bacterial cells, II) extraction and purification of the inclusion bodies, III) renaturation of the expressed protein, IV) ion-exchange chromatography, V) reverse-phase chromatography.
Owner:GEYMONAT
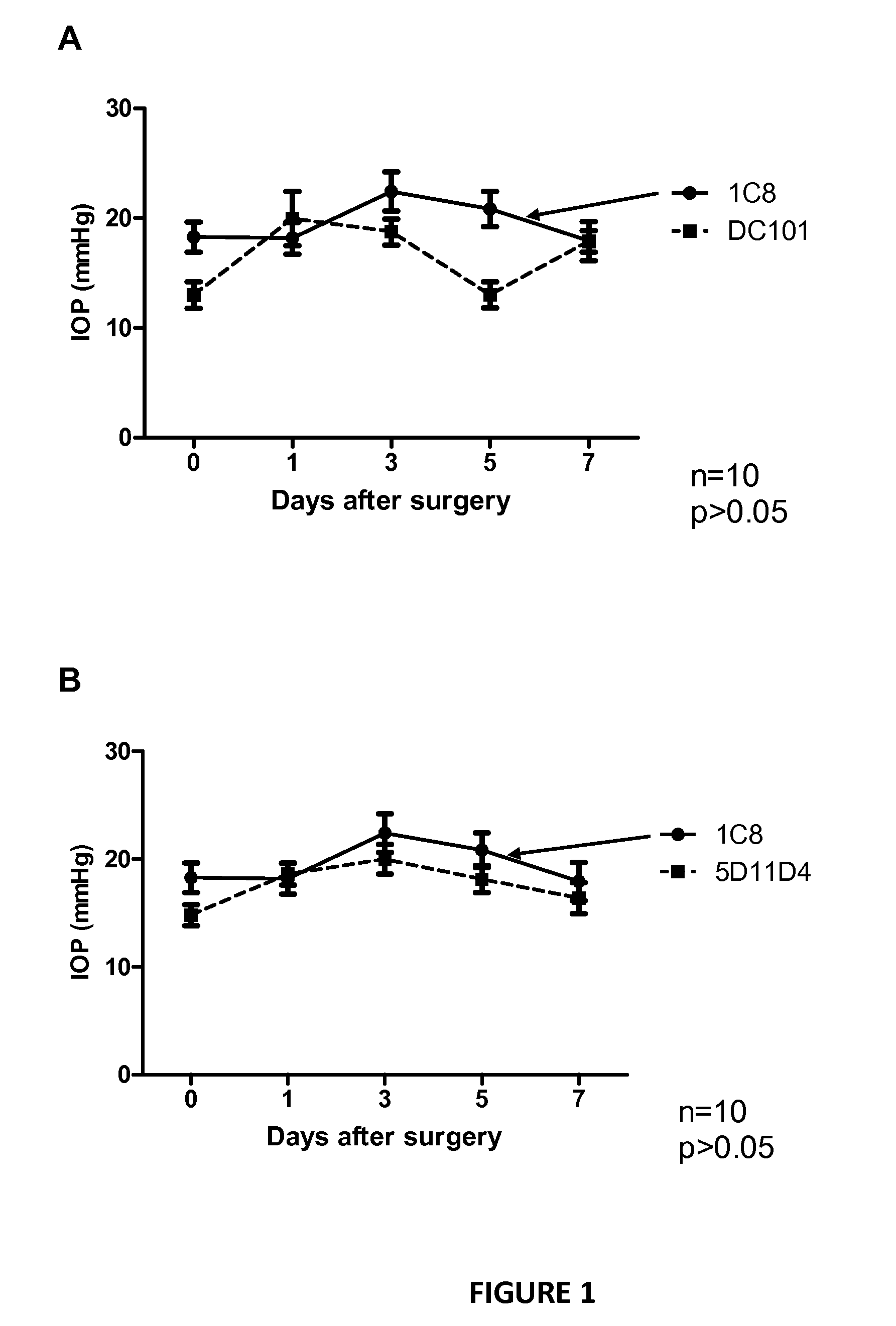
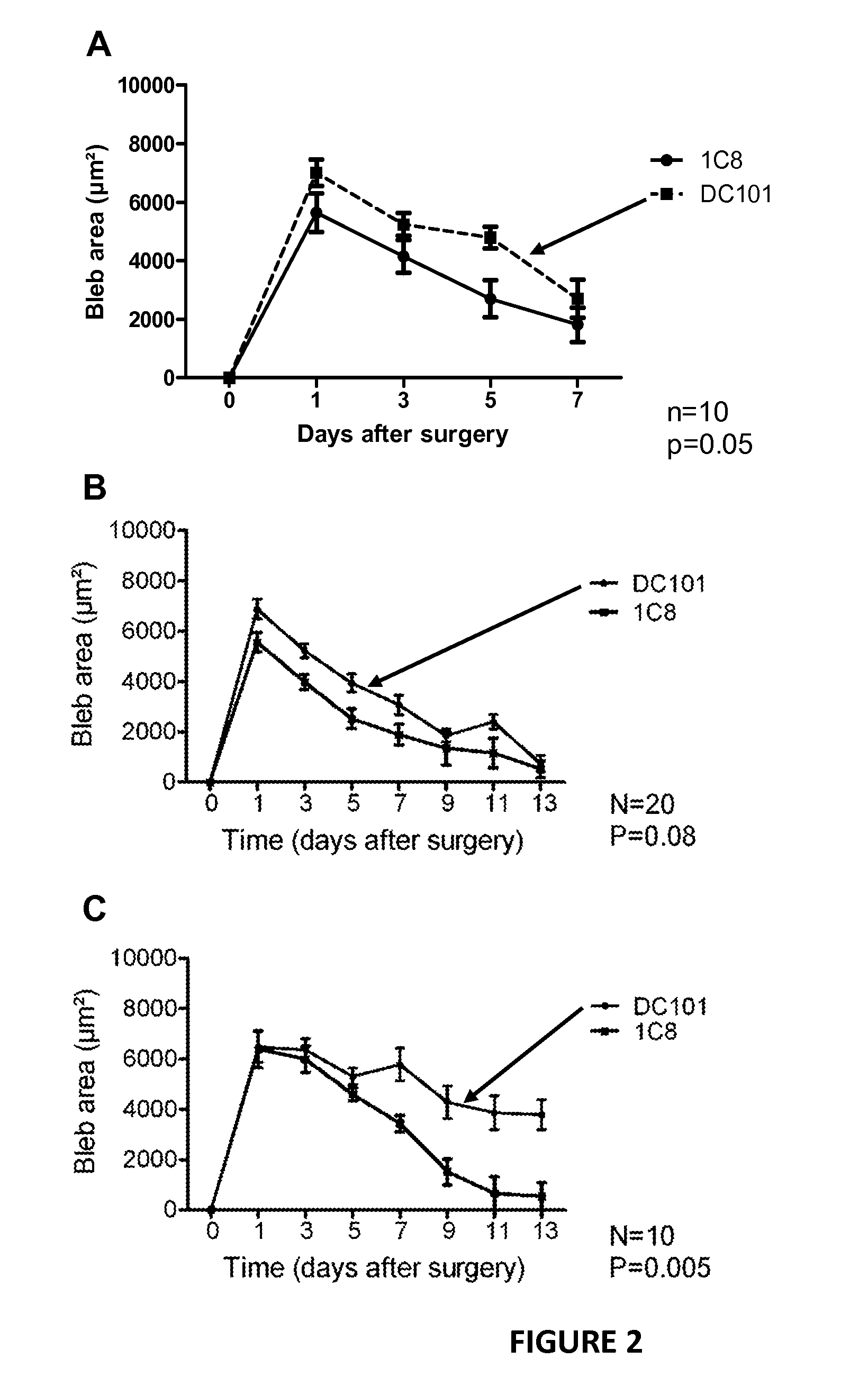
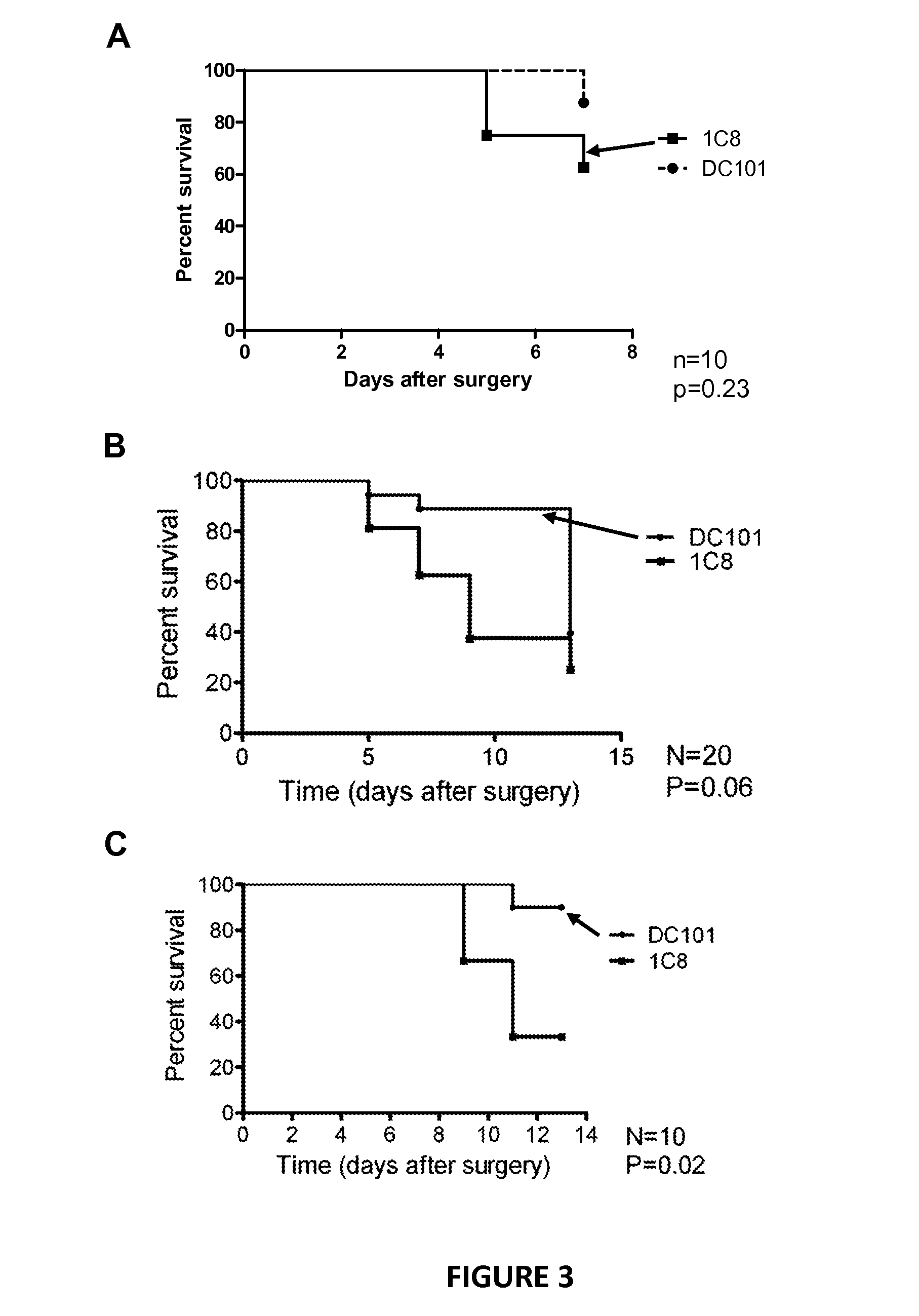
Improving trabeculectomy outcome by administering an anti-placental growth factor antibody
InactiveUS9089552B2Improving and enhancing success ratePreventing and reducing and retarding occurrenceSenses disorderPharmaceutical delivery mechanismPlacenta Growth FactorPlacenta
The current invention relates to the improvement of trabeculectomy surgery. The improvement more specifically resides in an extended lifetime of the sclera-corneal drainage channel created by trabeculectomy surgery. The improvement is obtained by post-surgical administration of an anti-PlGF antibody or fragment thereof.
Owner:OXURION NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com