Placenta growth factor quality control product and preparation method thereof
A technology for placental growth factor and quality control material, applied in biological testing, material testing products, biological material analysis and other directions, can solve the problems of difficult to obtain, difficult to widely apply, expensive and other problems, and achieves convenient operation, good stability and accuracy. high sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Use the ELISA analyzer to determine the value of the placental growth factor antigen, and the value concentration is 1.00mg / mL;
[0046] Placental growth factor quality control components are: newborn bovine serum, placental growth factor antigen, 2% casein, 2% glycerin, 2% Tween 80, 2% mannitol, 2% xylitol, 0.3% Proclin300, 0.3 %Proclin950;
[0047] The formulation steps were carried out as follows.
[0048] (1) Return the newborn bovine serum to room temperature, add it to the container, put it into the rotor, and place it on the mixer;
[0049] (2) Add casein, glycerin, Tween 80, mannitol, xylitol, Proclin300, and Proclin950 to the neonatal bovine serum returned to room temperature under stirring, and mix well to obtain a mixed solution;
[0050] (3) Determine that the quality control product preparation concentration is 32pg / mL, 100pg / mL, 1000pg / mL, take the placenta growth factor antigen and add it to the mixed solution in (2), and obtain the placenta growth fact...
Embodiment 2~12
[0054] Use the placenta growth factor antigen fixed value in embodiment 1;
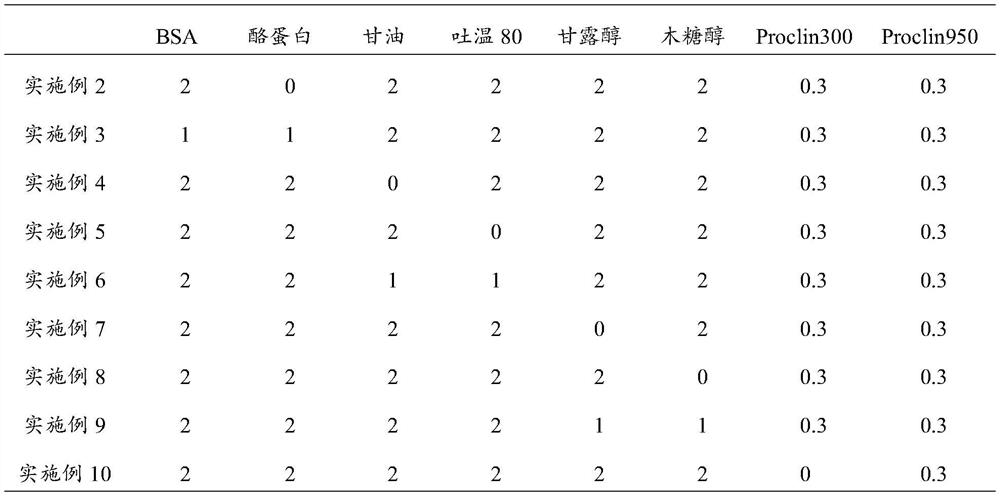
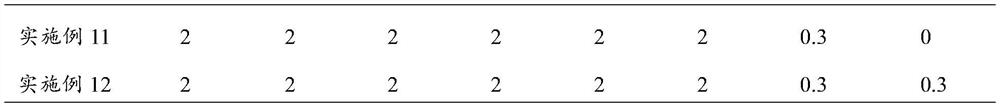
[0055] Components of placental growth factor quality control product: newborn bovine serum, placental growth factor antigen, and the ratio of other components are shown in Table 1:
[0056] Table 1 Components of Placenta Growth Factor Quality Control Products of Examples 2-12 (by weight percent)
[0057]
[0058]
[0059] Preparation steps are as in Example 1.
Embodiment 13
[0069] The quality control product prepared by embodiment 1-12 and comparative example 1-4 is carried out stability measurement
[0070] 1. Storage stability of quality control products at -20°C
[0071] The lyophilized products of placental growth factor quality control products with medium concentration levels prepared in Examples 1-12 and Comparative Examples 1-3 of the present invention were stored at -20°C, and stored at 0, 1, 2, 4, and 8, respectively. , 12, 16, 24, 36, 48, and 60 weeks, take one bottle for reconstitution, and use Hebei Twente Biotechnology Development Co. Test 3 times, and calculate the relative deviation between the mean and the target value, the results are shown in Table 2.
[0072] Table 2 Stability of different quality control products
[0073]
[0074]
[0075] The experimental results in Table 2 show that the quality control products of placenta growth factor prepared in Examples 1-12 of the present invention can be stored stably for 60 w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com