Polypeptides inhibiting neovascularization and uses thereof
a neovascularization and polypeptide technology, applied in the field of biomedicine, can solve the problems of many anatomical and functional barriers in eyes, unsatisfactory long-term efficacy, and destruction of local tissues, and achieve the effects of inhibiting the proliferation of huvecs, inhibiting the formation of lumens, and inhibiting endothelial cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis, Seperation and Purification of Small Peptide ZY3
[0121]The polypeptides ZY3 represented by SEQ ID NO: 1 were synthesized by using the commercially available SYMPHONY polypeptide synthesizer (12-channel, Protein Technologies. LLC., U.S.). The processes were as follows:
[0122]The reagents were calculated and prepared according to the software (Version. 201) of the polypeptide synthesizer. 2-Chlorotrityl Chloride Resin (Nankai Synthetic Technology Co., Ltd, Tianjin, China) was added into a reaction tubes, DMF (15 ml / g) (Dikma) was added and the tube was oscillated for 30 min. Solvents were suction filtered out through the sintered filter. 3-fold excess mole of Fmoc-L-OH (small peptide ZY3) amino acids (Suzhou Tianma Pharma Group Specialty Chemicals Co., Ltd.) was added and then 10-fold excess mole of DIEA (Sinopharm Shanghai Chemical Reagent Company) was added and finally, DMF was added for dissolution. The mixture was oscillated for 30 min. DMF was removed and 20% piperidine ...
example 2
Identification and Storage of Small Peptides ZY3
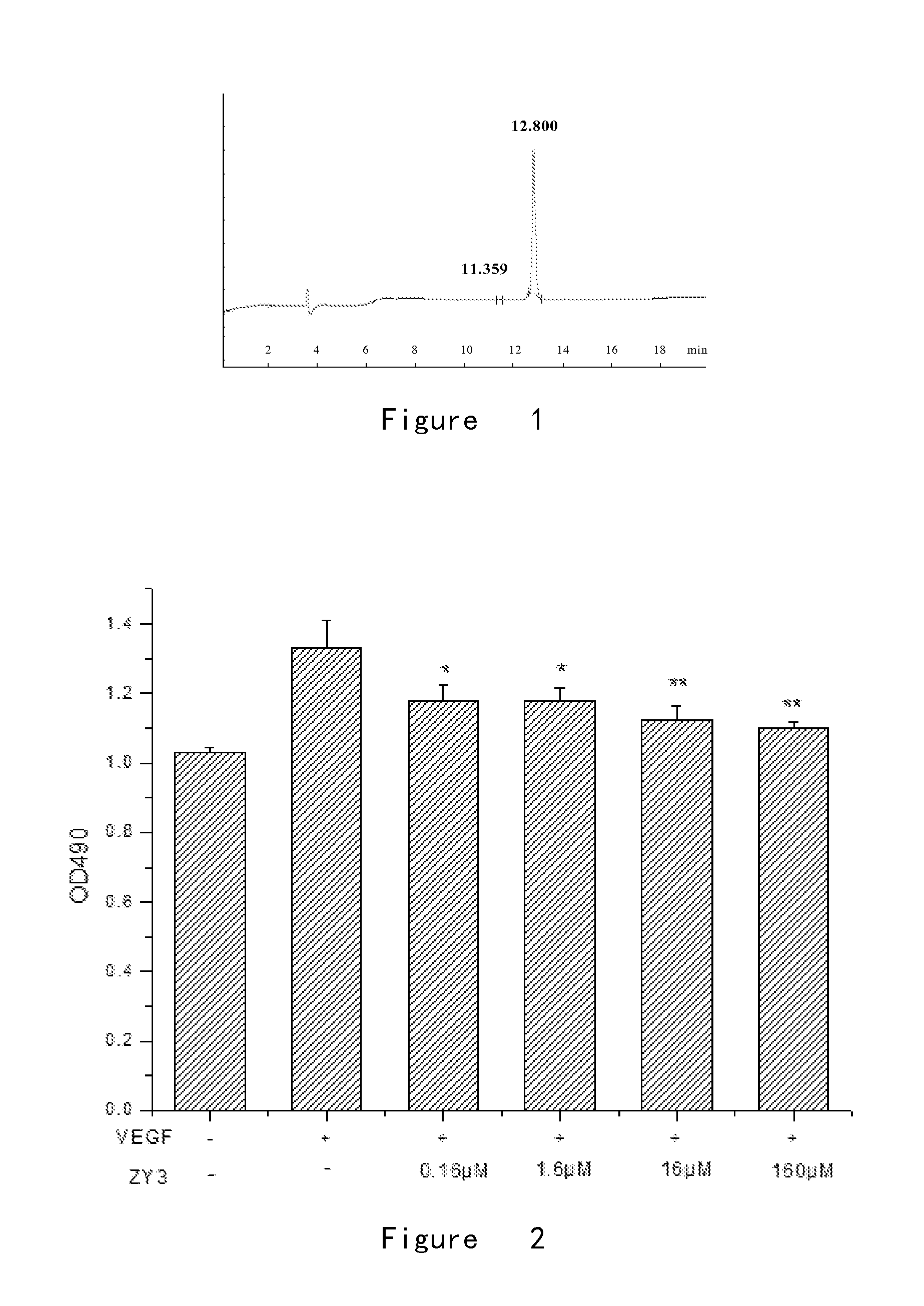
[0133]A small amount of small peptides ZY3 was taken for purity identification by HPLC analysis and molecular weight identification by ESI-MS.
[0134]The results showed that the elution peak of ZY3 was at 12.8 min with the purity over 99% (FIG. 1).
[0135]Small peptide ZY3 has 27 amino acids in total with a molecular weight of 3092.55. The small peptides in white powder form were sealed, packaged, and stored at −20° C.
example 3
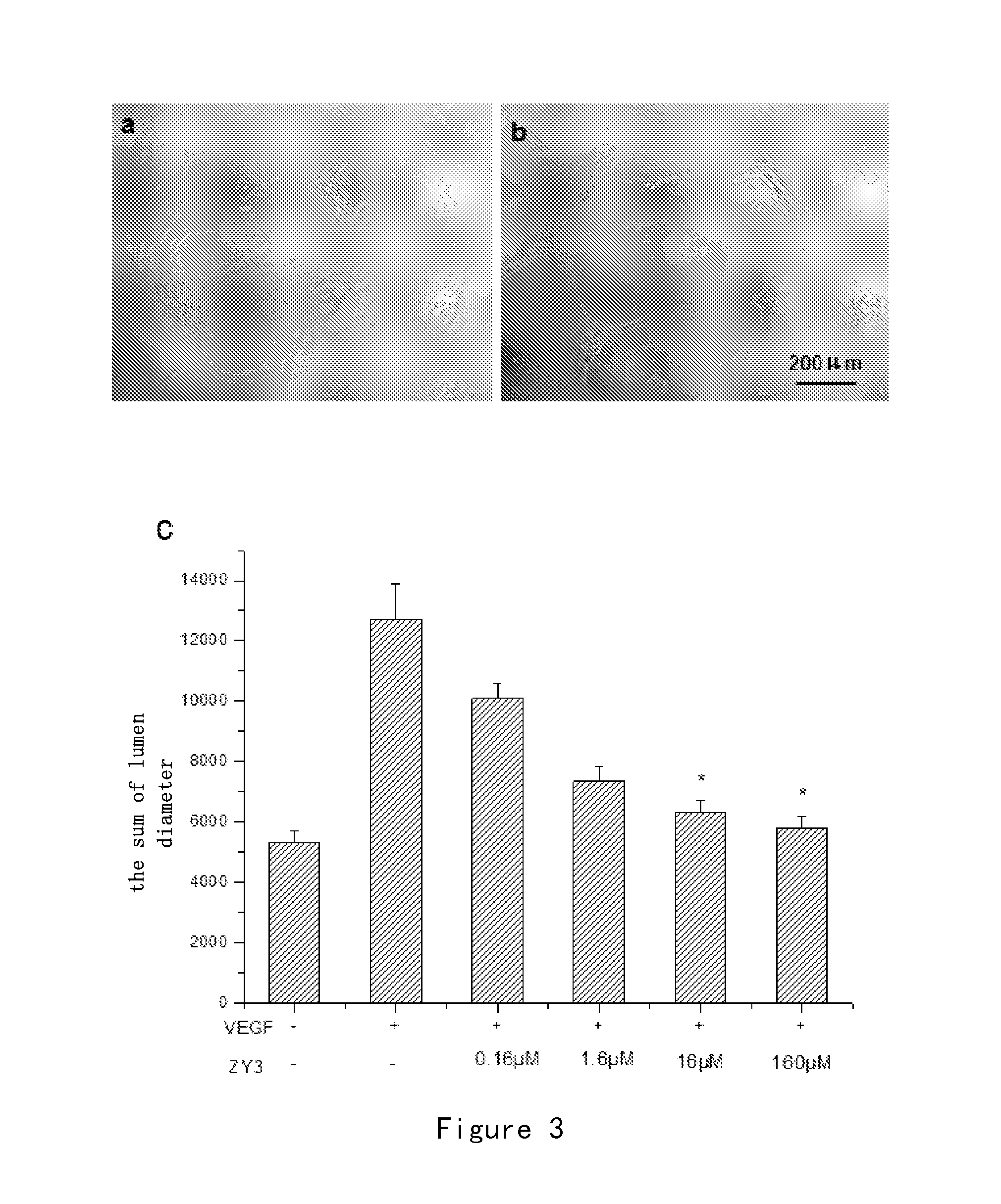
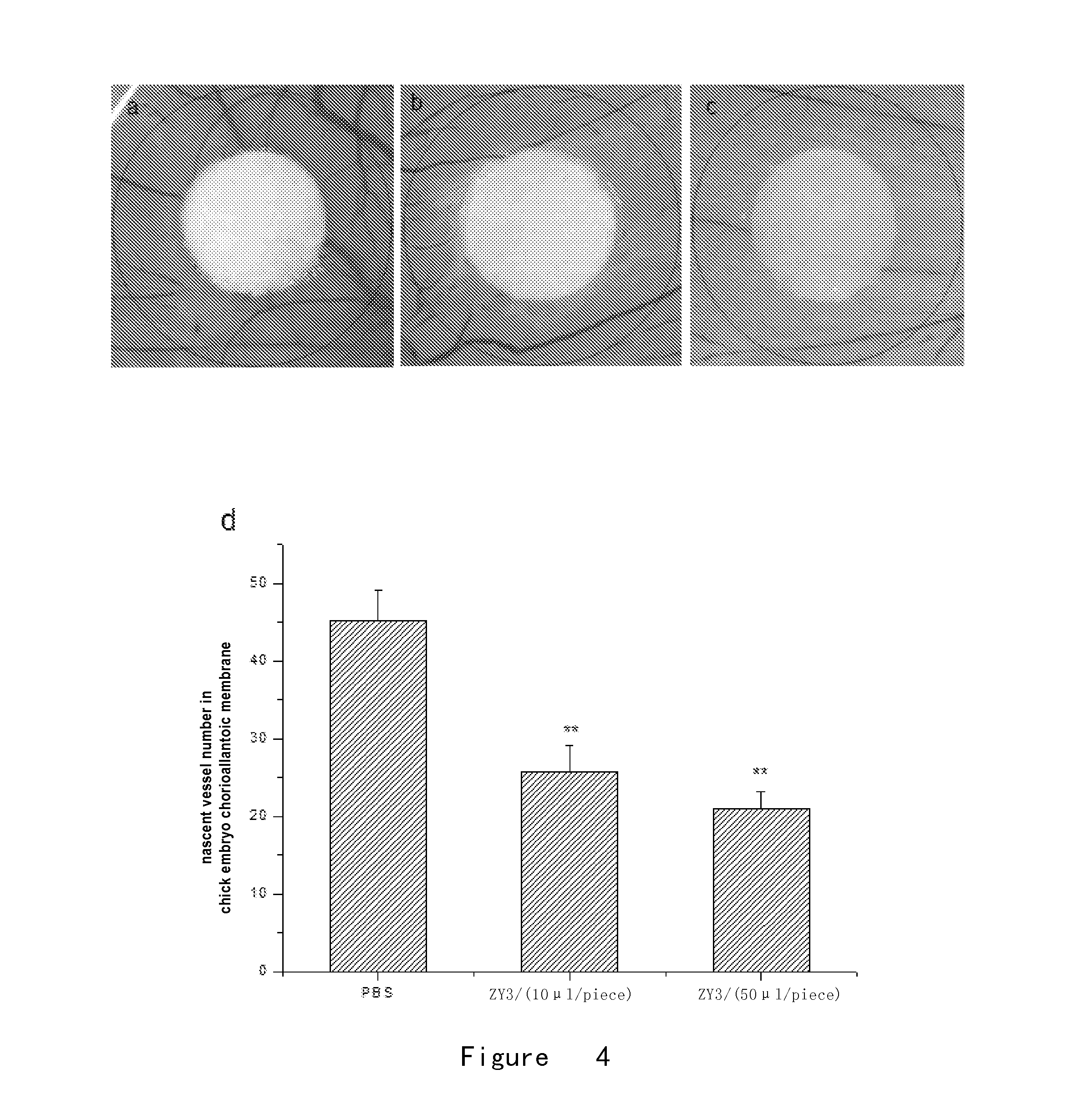
Effect of Small Peptides ZY3 on Proliferation Activity of HUVECs
[0136]The MTS method was used as follows:
[0137]Primary Human Umbilical Vein Endothelial Cells (HUVECs) (purchased from ScienCell Co.) were inoculated into a 96-well plate with an inoculation concentration of 2×104 / ml. After cells had adhered to the wall, serum-free culture medium ECM was added and the cells were cultivated at 37° C. for 24 hours. Then the serum-free culture medium ECM as negative control, VEGF (100 ng / ml) (purchased from Sigma Co.) as positive control, VEGF (100 ng / well)+small peptide ZY3 in different concentrations as treatment groups were added in each well. After a 24-hour incubation, 20 μl 1 MTS solution (purchased from Promega Corporation) was added in each well. After incubation at 37° C. for 4 hours, the absorbance in each well was measured at 490 nm by using microplate reader (Bio-Rad Co.). The proliferation activity of cells was determined according to OD490. Finally, SPSS11.0.1 was used for st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com