Method and device for preparing H2 by simultaneous thermochemical cycle mineralization of CO2 and decomposition of H2O
A thermochemical cycle, CO2 technology, applied in chemical instruments and methods, inorganic chemistry, hydrogen production, etc., can solve the problems of economy and low added value, and achieve easy large-scale industrial application, appropriate reaction temperature, high theoretical The effect of thermal efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
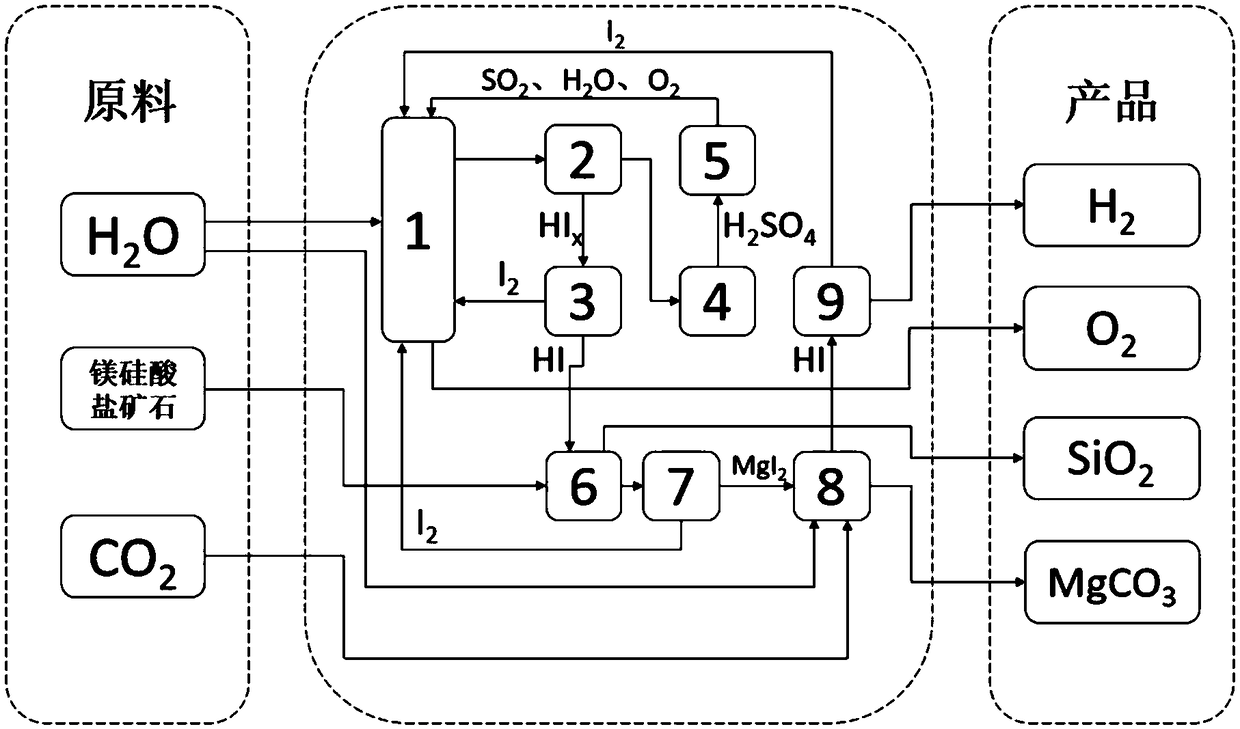
[0037] (1) 14mol of H 2 O, 1.5mol of I 2 and 1mol of SO 2 Send into the Bunsen reaction device 1, stir the reaction solution at a constant speed by the motor device to ensure that it is evenly mixed, and a spontaneous exothermic reaction occurs at 20 ° C and 1 atm, producing a watery HI phase (HI x ) and H 2 SO 4 Phase solution, in which the HI phase mainly contains hydrogen iodide solution and excess iodine, H 2 SO 4 Phase mainly contains H 2 SO 4 Solution, the chemical reaction formula of this reaction is as follows:
[0038] I 2 +SO 2 +2H 2 O→2HI+H 2 SO 4
[0039] The biphasic solution in the Bunsen reaction unit 1 is separated in the liquid phase separation unit 2;
[0040] (2) Under 120°C, 0.08atm and adiabatic conditions, in H 2 SO 4 The separated H in the concentration unit 4 2 SO 4 The phase solution is subjected to multi-stage sulfuric acid concentration treatment;
[0041] (3) Concentrated H 2 SO 4 phase into concentrated H 2 SO 4 In the cataly...
specific Embodiment 2
[0052] (1) 15molH 2 O, 5molI 2 and 1molSO 2 Send into the Bunsen reaction device 1, stir the reaction solution at a constant speed by the motor device to ensure that it is evenly mixed, and an autonomous exothermic reaction occurs at 70 ° C and 1.5 atm to produce a watery HI phase (HI x ) and H 2 SO 4 Phase solution, in which the HI phase mainly contains hydrogen iodide solution and excess iodine, H 2 SO 4 Phase mainly contains H 2 SO 4 Solution, the chemical reaction formula of this reaction is as follows:
[0053] I 2 +SO 2 +2H 2 O→2HI+H2 SO 4
[0054] The biphasic solution in the Bunsen reaction unit 1 is separated in the liquid phase separation unit 2;
[0055] (2) Under 190°C, 0.69atm and adiabatic conditions, in H 2 SO 4 The separated H in the concentration unit 4 2 SO 4 The phase solution is subjected to multi-stage sulfuric acid concentration treatment;
[0056] (3) Concentrated H 2 SO 4 phase into concentrated H 2 SO 4 In the catalytic decomposit...
specific Embodiment 3
[0067] (1) 16molH 2 O, 9molI 2 and 1molSO 2 Send into the Bunsen reaction device 1, stir the reaction solution at a constant speed by the motor device to ensure that it is evenly mixed, and an autonomous exothermic reaction occurs at 120 ° C and 2 atm to produce a watery HI phase (HI x ) and H 2 SO 4 Phase solution, in which the HI phase mainly contains hydrogen iodide solution and excess iodine, H 2 SO 4 Phase mainly contains H 2 SO 4 Solution, the chemical reaction formula of this reaction is as follows:
[0068] I 2 +SO 2 +2H 2 O→2HI+H 2 SO 4
[0069] The biphasic solution in the Bunsen reaction unit 1 is separated in the liquid phase separation unit 2;
[0070] (2) Under 260°C, 1.3atm and adiabatic conditions, in H 2 SO 4 The separated H in the concentration unit 4 2 SO 4 The phase solution is subjected to multi-stage sulfuric acid concentration treatment;
[0071] (3) Concentrated H 2 SO 4 phase into concentrated H 2 SO 4 In the catalytic decomposit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com