A Zn-Cr hydrotalcite-derived Ni-based catalyst for hydrogen production by autothermal reforming of acetic acid
A nickel-based catalyst, autothermal reforming technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, heterogeneous catalyst chemical elements, etc., can solve catalyst deactivation, intolerance Solve problems such as sintering and poor stability, and achieve the effects of stable skeleton structure, improved oxidation resistance, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] Weigh 2.398g of Ni(NO 3 ) 2 ·6H 2 O, 5.213g of Zn(NO 3 ) 2 ·6H 2 O and 10.311g of Cr(NO 3 ) 3 9H 2 O, add 51.5ml of deionized water to prepare solution #1; weigh 8.246g of NaOH and 1.366g of anhydrous Na 2 CO 3 , add 219.0ml of deionized water to prepare solution #2; at 78°C and the pH of the solution at 10.5±0.5, add solution #1 and solution #2 dropwise into the beaker and keep stirring for co-precipitation reaction, And continue stirring and aging for 14h; after aging, the mixture is suction filtered and washed 3 times, and the resulting precipitate is dried in a 105°C drying oven for 12 hours, and the XRD characterization shows that no hydrotalcite structure is formed; the precipitate is roasted at 700°C for 4 hours A CUT-ZNC-101 catalyst was obtained after 1 hour. The molar composition of the catalyst is (ZnO) 0.5 (NiO) 0.16 (CrO 1.5 ) 0.34 , The weight percentage is: zinc oxide is 35.6%, nickel oxide is 15.4%, and chromium oxide is 49.0%.
[0029] Th...
example 2
[0032] Weigh 2.361g of Ni(NO 3 ) 2 ·6H 2 O, 10.410g of Zn (NO 3 ) 2 ·6H 2 O and 2.875g of Cr(NO 3 ) 3 9H 2 O, add 50.3ml of deionized water to prepare solution #1; weigh 8.047g of NaOH and 1.333g of anhydrous Na 2 CO 3 , adding 213.8ml of deionized water to prepare solution #2; the subsequent steps were the same as those in Reference Example 1 to obtain the CUT-ZNC-102 catalyst. The molar composition of the catalyst is (ZnO) 0.68 (NiO) 0.18 (CrO 1.5 ) 0.14 , The weight percentage is: zinc oxide is 69.2%, nickel oxide is 17.1%, and chromium oxide is 13.7%.
[0033] The activity of the CUT-ZNC-102 catalyst was investigated by the autothermal reforming reaction of acetic acid. The space velocity was 11250mL / (g-catalyst h), the reaction temperature was 700°C, and the feed ratio was CH 3 COOH / H 2 O / O 2 =1 / 4.0 / 0.28, the reaction time is 10h; the acetic acid conversion rate of the catalyst is about 99.5%, and the hydrogen production rate is 2.50molH 2 / molHAc, CO 2...
Embodiment 1
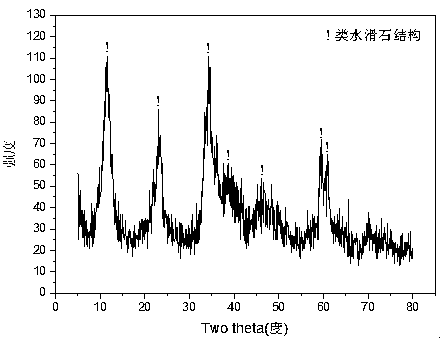
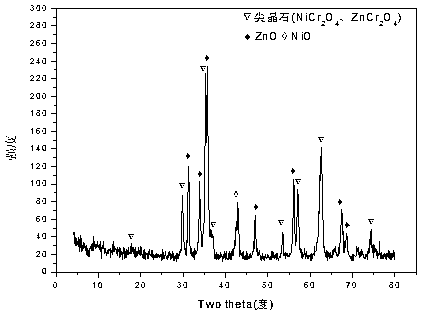
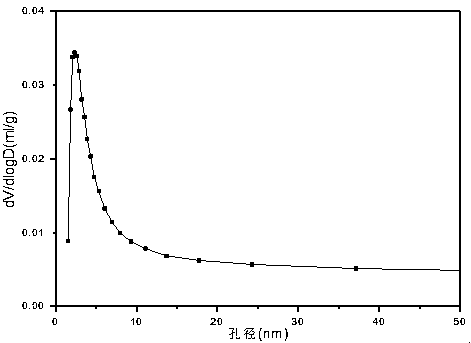
[0035] Weigh 2.432g of Ni (NO 3 ) 2 ·6H 2 O, 8.820g of Zn(NO 3 ) 2 ·6H 2 O and 5.070g of Cr(NO 3 ) 3 9H 2 O, add 50.7ml of deionized water to prepare solution #1; weigh 16.217g of NaOH and 2.686g of anhydrous Na 2 CO 3 , add 430.8ml of deionized water to prepare solution #2; the follow-up steps are the same as those in Reference Example 1 to obtain the precursor of Zn-Cr carbonate-type hydrotalcite structure, and its typical structure is shown in the attached figure 1 shown; after roasting, a zinc oxide-containing spinel phase (NiCr 2 o 4 / ZnCr 2 o 4 ) Zn-Ni-Cr-O composite oxide, its typical structure is as attached figure 2 As shown, the CUT-ZNC-103 catalyst is obtained; the catalyst is a mesoporous material, and its typical pore size distribution is shown in the attached image 3 As shown, the pores are concentrated in the range of 2-10 nm. The molar composition of the catalyst is (ZnO) 0.59 (NiO) 0.16 (CrO 1.5 ) 0.25 , The weight percentage is: zinc oxi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com