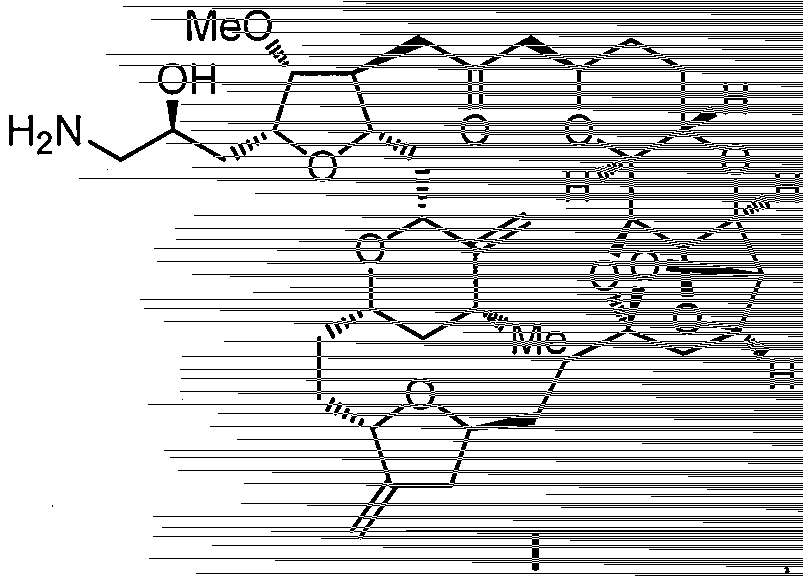
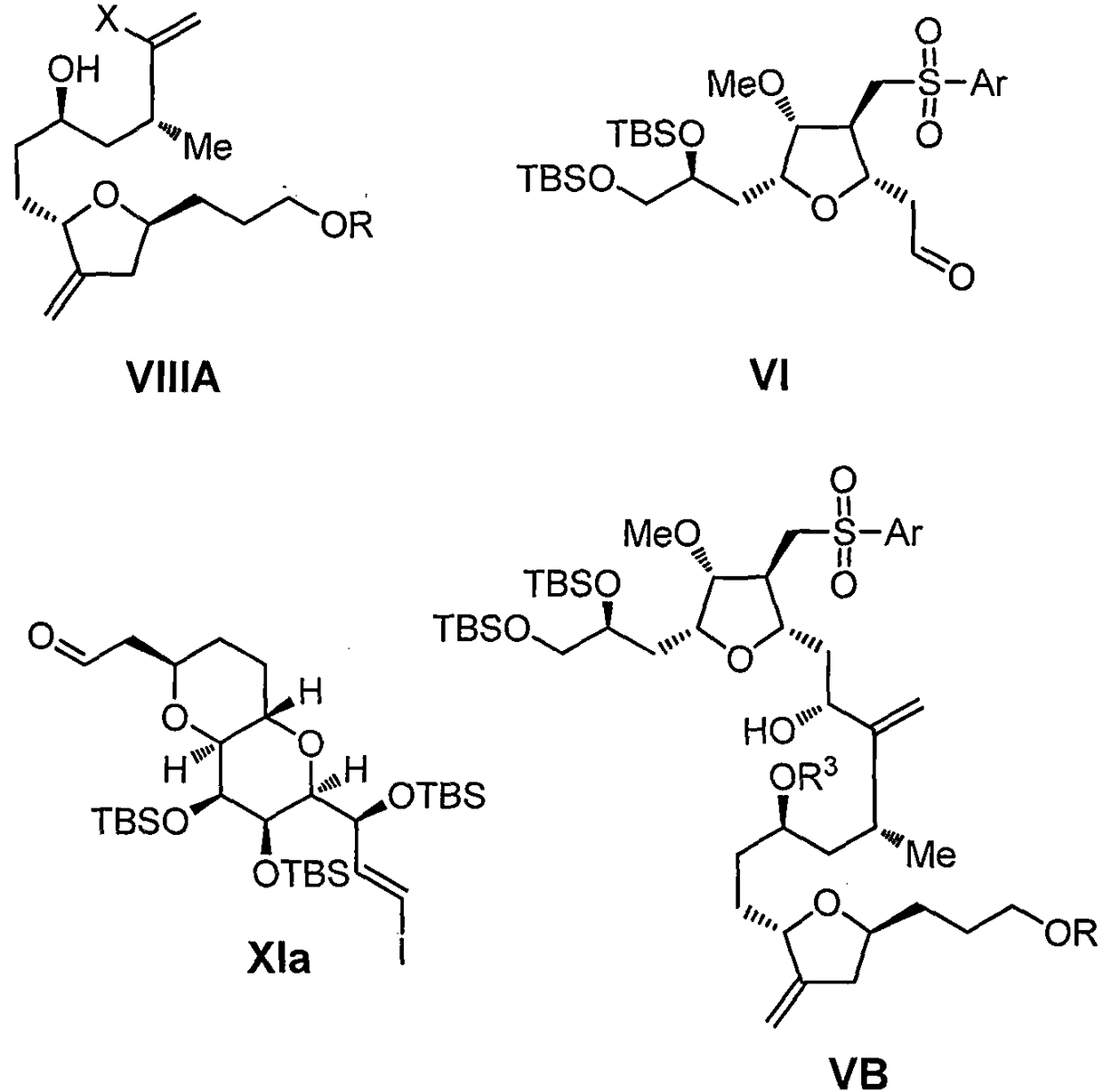
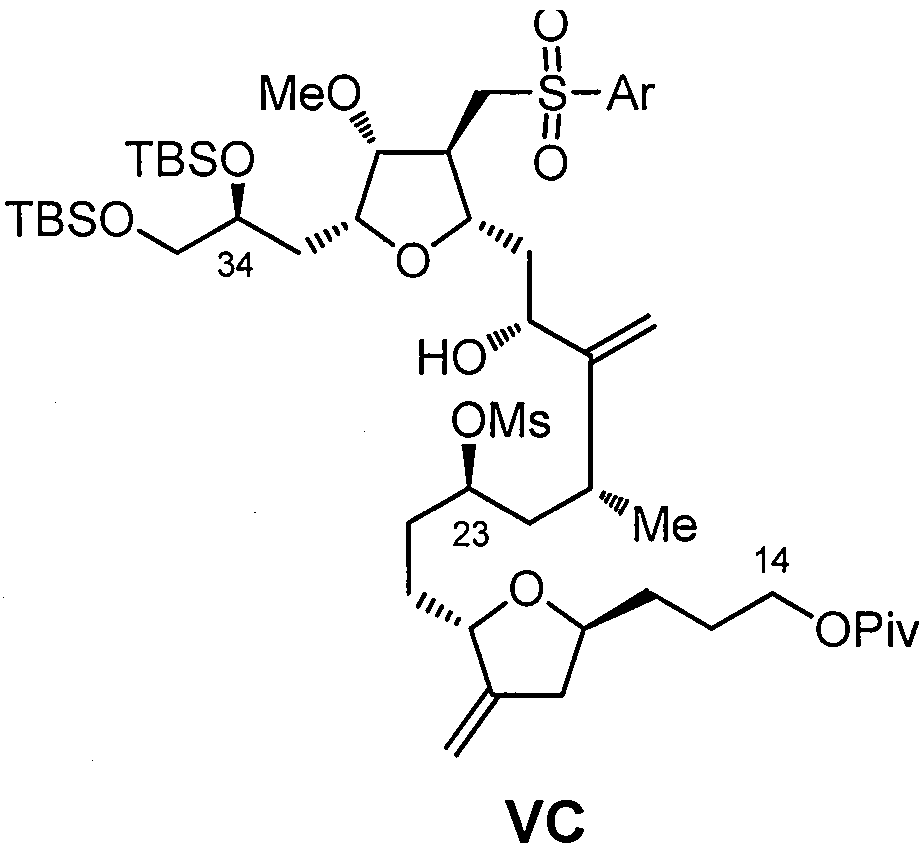
Eribulin intermediates and preparation method thereof
一种化合物、定义的技术,应用在化学仪器和方法、有机化学、散装化学品生产等方向,达到反应条件温和、合成路线绿色高效、合成收率高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0298] Embodiment 1: preparation compound VIIa
[0299] To a solution of Villa (8.17 g, 18 mmol) in THF (150 mL) at room temperature was first added Et 3 N (16.2 g) was then slowly added MsCl (12.1 g). After the addition was complete, the reaction continued to stir under these conditions for 1 h. After the completion of the reaction as detected by TLC, the reaction solution was quenched with saturated aqueous sodium bicarbonate solution, extracted with ethyl acetate, and the concentrated solution was purified by column to obtain compound VIIa (8.76 g).
[0300] MS (ESI) m / z: 515 (M+H + )
[0301] 1 H NMR (400MHz, CDCl 3 ): δ6.36(d, J=1.2Hz, 1H), 5.84(d, J=1.6Hz, 1H), 5.01-4.99(m, 1H), 4.90-4.85(m, 2H), 4.72-4.65( m, 1H), 4.41-4.35(m, 1H), 4.08-4.01(m, 1H), 3.98-3.94(m, 2H), 3.87-3.83(m, 2H), 3.02(s, 3H), 2.71- 2.64(m, 1H), 2.31-2.25(m, 1H), 2.10-2.02(m, 1H), 1.94-1.75(m, 4H), 1.73-1.51(m, 6H), 1.00(d, J=6.4 Hz, 3H).
Embodiment 2
[0302] Embodiment 2: preparation compound VIIb
[0303] To a solution of VIIIb (1.02g, 2.1mmol) in dichloromethane (15mL) was added pyridine (2.3g) at -30°C, followed by the slow addition of Ms 2 O (1.59 g). After the addition was complete, the reaction continued to stir under these conditions for 3h. After the completion of the reaction as detected by TLC, the reaction solution was quenched with saturated aqueous sodium bicarbonate solution, extracted with ethyl acetate, and the concentrated solution was purified by column to obtain compound VIIb (1.06 g).
[0304] MS (ESI) m / z: 529 (M+H + )
Embodiment 3
[0305] Embodiment 3: preparation compound VIIc
[0306] To a solution of VIIIc (0.68g, 1.3mmol) in ethyl acetate (15mL) was added 2,6-lutidine (2.1g) at -10°C, followed by the slow addition of Ms 2 O (1.06g). After the addition was complete, the reaction continued to stir under these conditions for 30 min. After the completion of the reaction as detected by TLC, the reaction solution was quenched with saturated aqueous sodium bicarbonate solution, extracted with ethyl acetate, and the concentrated solution was purified by column to obtain compound VIIc (0.72 g).
[0307] MS (ESI) m / z: 557 (M+H + )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com