A Method for Preparation of Thymopentin Molecularly Imprinted Hydrogels by Wave Polymerization
A technology of molecular imprinting and hydrogel, which is applied in the field of wave polymerization to prepare thymopentin molecular imprinting hydrogel, to achieve the effects of good selectivity and imprinting effect, low cytotoxicity and large drug loading capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
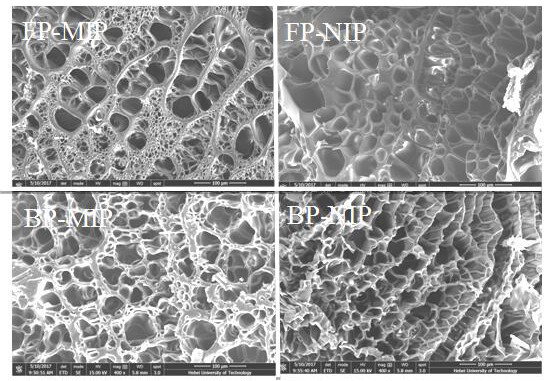
[0035] In order to investigate the surface morphology of thymopentin molecularly imprinted hydrogels more clearly, we used wave polymerization to prepare molecularly imprinted hydrogels (FP-MIP) and non-imprinted hydrogels (FP-NIP), and thermal polymerization to prepare molecules Imprinted hydrogel (BP-MIP) and non-imprinted hydrogel (BP-NIP). The hydrogel material was analyzed by field emission scanning electron microscopy to characterize the structural characteristics of the hydrogel. The specific operation steps are as follows:
[0036] a. Preparation of Thymopentin Molecularly Imprinted Hydrogel Drug Carrier Materials by Wave Polymerization:
[0037] 1) Preparation of DES system: Mix choline chloride (4g) and ethylene glycol (10.8mL) according to the measured amount, stir magnetically in an oil bath at 80°C until a clear and uniform solution is obtained, and store at room temperature for later use.
[0038] 2) Mix thymopentin (326mg), N-isopropylacrylamide (670mg), acryl...
Embodiment 2
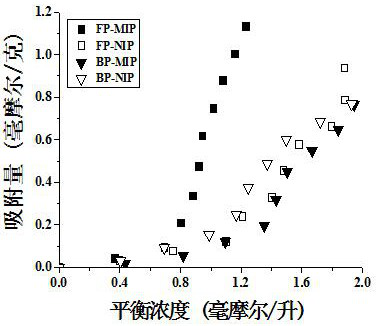
[0051] The thymopentin equilibrium adsorption experiment was used to study the specific adsorption performance of the molecularly imprinted hydrogel on the template molecule thymopentin. In order to investigate the specific recognition ability of the imprinted hydrogel on thymopentin, the thymopentin molecular Adsorption isotherms of hydrogel and non-imprinted hydrogel in the range of 0.5-5.0mmol / L. The specific operation steps are as follows:
[0052] a. Same as above method (Example 1) Synthetic Wave Polymerized Molecularly Imprinted Hydrogel (FP-MIP), Wave Polymerized Non-imprinted Hydrogel (FP-NIP), Thermal Polymerized Molecularly Imprinted Hydrogel (BP-MIP), Thermally polymerized non-imprinted hydrogels (BP-NIP).
[0053] b. Weigh 10.0 mg of dried hydrogel material and put them into 5ml centrifuge tubes. Then prepare an aqueous solution of 0.5-5.0mmol / L TP-5, add 3.0mL of TP-5 solutions of different concentrations to the above centrifuge tubes, soak in a water bath at 3...
Embodiment 3
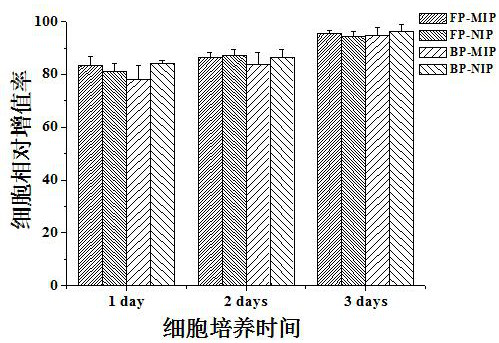
[0060] MTT assay to study the cytotoxicity of hydrogel materials. In order to investigate the cytocompatibility of the hydrogel material, the relative cell proliferation rate after the cells were cultured on the hydrogel material extract for different periods of time was determined. The specific operation steps are as follows:
[0061] a. Same as above method (Example 1) Synthetic Wave Polymerized Molecularly Imprinted Hydrogel (FP-MIP), Wave Polymerized Non-imprinted Hydrogel (FP-NIP), Thermal Polymerized Molecularly Imprinted Hydrogel (BP-MIP), Thermally polymerized non-imprinted hydrogels (BP-NIP).
[0062] b. Take mouse fibroblasts grown in logarithmic phase, trypsinize, and dilute to 1×10 with DMEM medium containing 10% fetal bovine serum (FBS). 4 Concentration, add to 96-well plate, add 100 μL of cell suspension to each well, and then place the cells in a cell culture incubator (37°C, 5% CO 2 ) for 24 hours, the original culture medium was discarded, and the hydrogel ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com