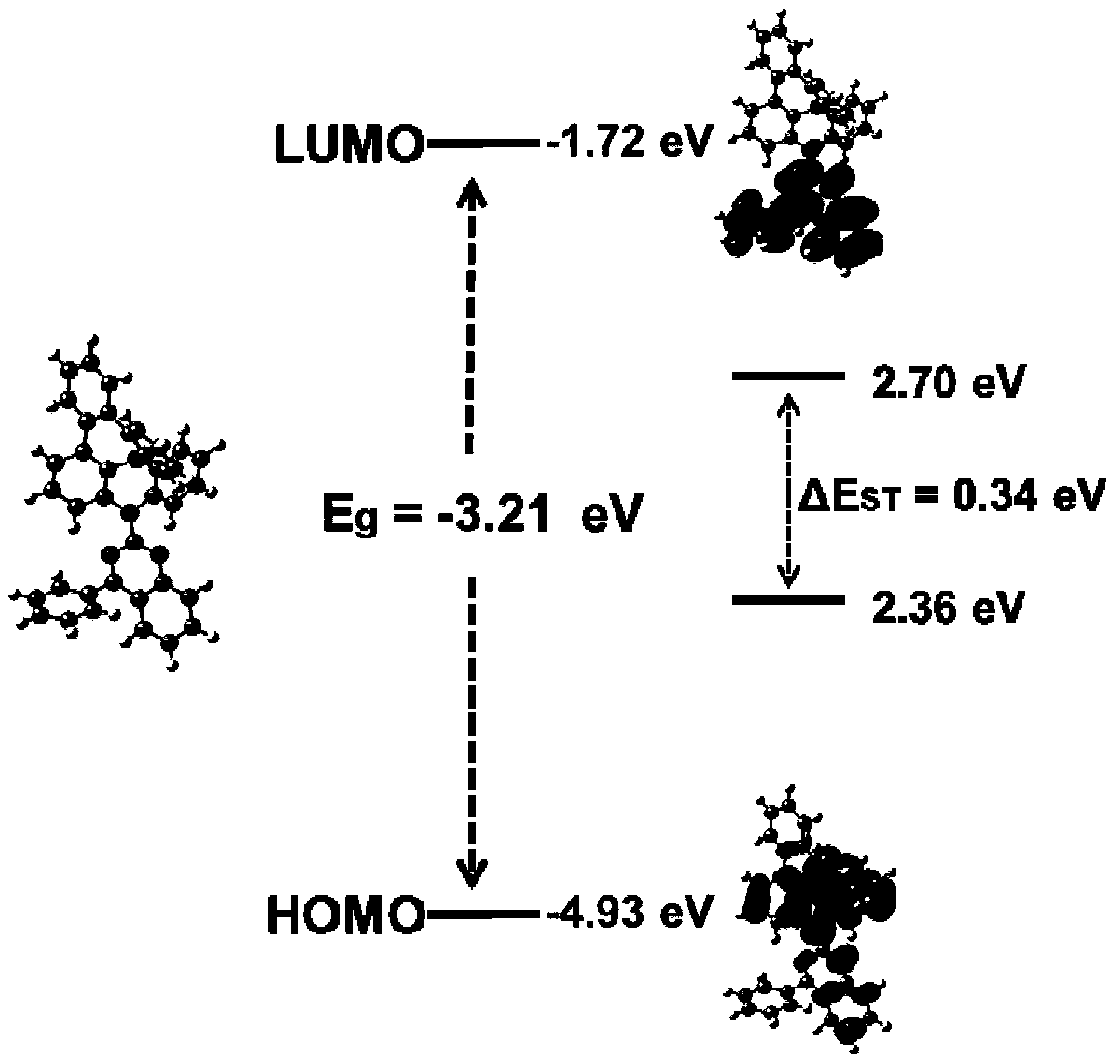
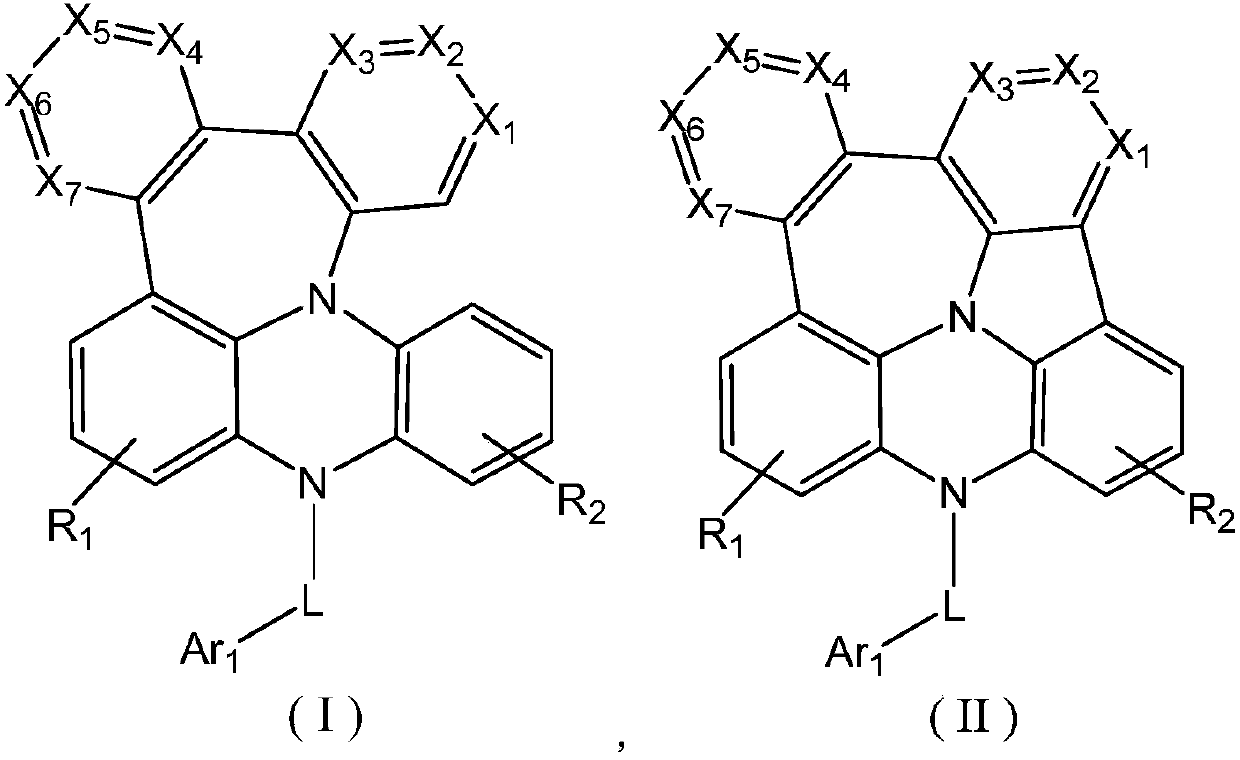
Fused ring compound as well as preparation method and application thereof
A compound and condensed ring technology, applied in the field of condensed ring compounds and their preparation, can solve the problems of inefficient energy transfer of host materials, unsatisfactory light-emitting area, and low light-emitting efficiency and light-emitting performance of devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The present embodiment provides a fused ring compound having the structure shown in the following formula D-2:
[0075]
[0076] The synthetic route of the fused ring compound represented by formula D-2 is as follows:
[0077]
[0078] The preparation method of the fused ring compound shown in formula D-2 specifically comprises the following steps:
[0079] (1) Synthesis of Intermediate 1-1
[0080] Under nitrogen protection, into a 500mL three-necked flask, add 9.1g (50mmol) of the compound represented by formula (A-1), 7.8g (25mmol) of 2,2'-dibromobiphenyl (formula (B-1) the compound shown), 200 mL of tetradioxane, 48 mg of cuprous iodide (0.25 mmol), 3.5 g of sodium tert-butoxide (36 mmol), 0.3 mL of cis-1,2-cyclohexanediamine, 100°C After the reaction was performed for 12 hours, after extraction with chloroform three times, the solvent was removed by rotary evaporation, and passed through a silica gel column to obtain 6.2 g of solid intermediate 1-1 (yield: ...
Embodiment 2
[0087] The present embodiment provides a fused ring compound having the structure shown in the following formula D-1:
[0088]
[0089] The synthetic route of the fused ring compound represented by formula D-1 is as follows:
[0090]
[0091] The preparation method of the fused ring compound represented by formula D-1 specifically comprises the following steps:
[0092] (1) with the synthetic method shown in embodiment 1, synthesize intermediate 2-1;
[0093] (2) Synthesis of fused ring compound D-1
[0094] Under nitrogen protection, add 1.7g compound 1-2 (5mmol), 0.03g palladium acetate (0.15mmol), 0.1g tri-tert-butylphosphine (0.55mmol), 2g compound (5.1mmol), 1.41g of sodium tert-butoxide, 750mL of toluene, reacted at 110°C for 12 hours, cooled to room temperature, extracted with chloroform, evaporated to remove the solvent, and passed through a silica gel column to obtain 2.6g of solid compound C-16 (yield 81%) ).
[0095] Elemental analysis: (C45H29N5) Theoret...
Embodiment 3
[0097] The present embodiment provides a fused ring compound having the structure shown in the following formula D-7:
[0098]
[0099] The synthetic route of the fused ring compound represented by formula D-7 is as follows:
[0100]
[0101] The preparation method of the fused ring compound represented by formula D-7 specifically includes the following content:
[0102] (1) Synthesis of intermediate 3-1
[0103] Under nitrogen protection, into a 500mL three-necked flask, add 5.6g of the compound (20mmol) of formula (C-1), 3.5g of 3-chloro-2-fluoronitrobenzene (compound of formula (E-1)) (20mmol) ), 7.8g of cesium carbonate (24mmol), 80mL of dimethyl sulfoxide, reacted for 15 hours, extracted with toluene, evaporated to remove the solvent, passed through a silica gel column to obtain 6.5g of solid intermediate 3-1 (yield 75%);
[0104] (2) Synthesis of intermediate 4-1
[0105] Under nitrogen protection, 4.3 g of intermediate 3-1 (10 mmol), 0.2 g of palladium acetate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com