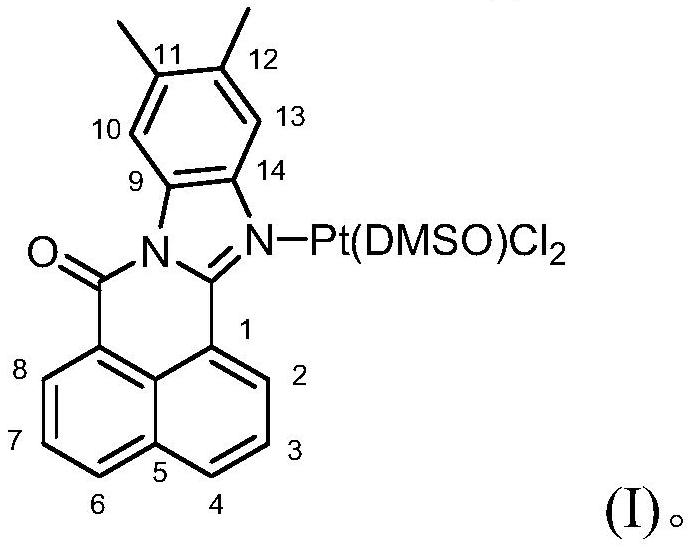
11,12-Dimethylbenzimidazole-1,8-naphthalimide-platinum complex and its preparation method and application
A technology of dimethylformamide and compound, applied in the field of medicine, can solve the problems that have not been seen before, and achieve the effects of stable quality, high yield and short preparation period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Measure 11,12-dimethylbenzimidazole-1,8-naphthalimide (100mg, 0.3354mmol), dichlorobis(dimethylsulfoxide) platinum(II) (Pt(DMSO) 2 Cl 2 ) (141.20mg, 0.3354mmol), 5ml of methanol and 5ml of chloroform were placed in a round-bottomed flask, and the temperature was 60°C, and the reaction was stirred for 48h. A yellow solid precipitated out and was collected and dried to obtain 130 mg of a yellow powder product with a yield of 60.46%.
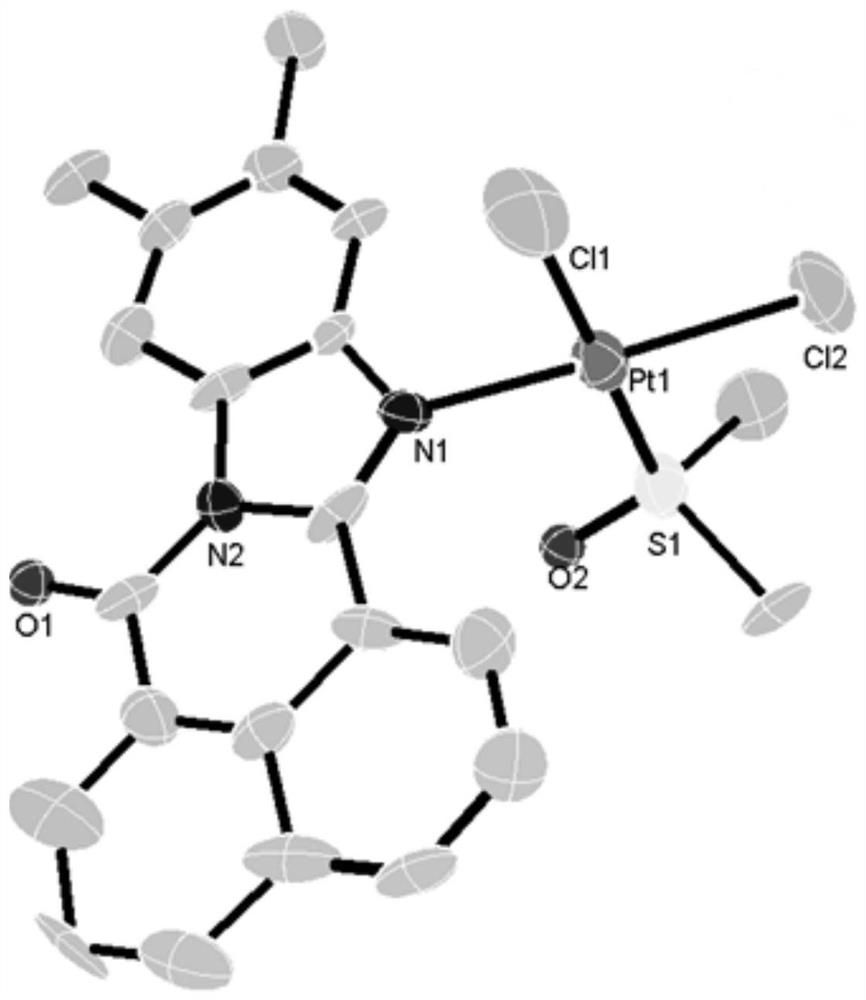
[0030] Characterizations such as mass spectrometry, elemental analysis and X-single crystal diffraction are carried out to the product obtained in this implementation, as follows:
[0031] (1) MS m / z:685.0704[M-Cl+DMSO] + .
[0032] (2) Anal. Calc. (for C 22 h 20 Cl 2 N 2 o 2 PtS) C 41.13; H 3.14; N 4.36%, Found. C 41.33; H 3.17; N 4.35%.
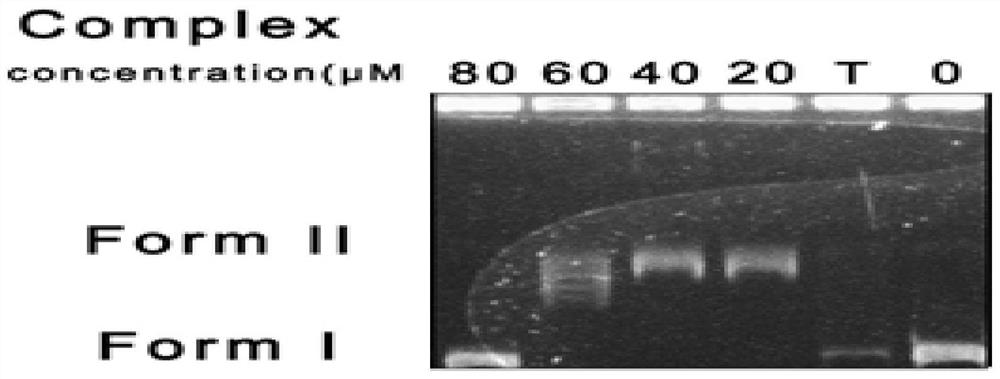
[0033] (3) Take 12 mg of the product obtained in this practice and 5 ml of methanol / chloroform mixed solution (the volume ratio of methanol and chloroform is 1:1) and place it in a sealed tube, r...
Embodiment 2
[0042] Take 11,12-dimethylbenzimidazole-1,8-naphthalimide (298.3mg, 1mmol), Pt(DMSO) 2 Cl 2 (422.9mg, 1mmol) and 9ml of methanol were placed in a thick-walled pressure-resistant bottle. After dissolving, they were stirred and reacted for 36h at a temperature of 70°C, and naturally cooled to room temperature. A yellow solid precipitated, separated, and dried to obtain a yellow powder 434.45 mg, yield 60.30%.
[0043] Mass spectrometry, elemental analysis and further X-ray single crystal diffraction analysis were performed on the product obtained in this example, and it was determined that the product obtained in this example was the target compound.
Embodiment 3
[0045] Take 11,12-dimethylbenzimidazole-1,8-naphthalimide (220.8mg, 0.7404mmol), Pt(DMSO) 2 Cl 2 (311.64mg, 0.7404mmol) and 5ml DMSO were placed in a round-bottomed flask, dissolved and stirred at 80°C for 24 hours, cooled to room temperature naturally, a yellow solid precipitated, separated and dried to obtain 294.22mg of a yellow powder , yield 55.26%.
[0046] Mass spectrometry, elemental analysis and further X-ray single crystal diffraction analysis were performed on the product obtained in this example, and it was determined that the product obtained in this example was the target compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com