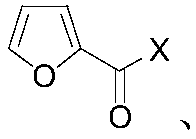
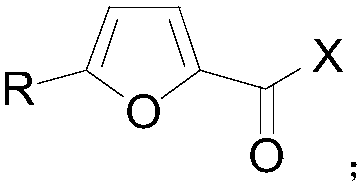
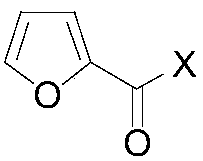
Method for preparing 2,5-disubsituted furan compound
A compound and disubstitution technology, applied in the field of preparation of furan compounds, can solve the problems of harsh reaction conditions, high synthesis cost, and long steps of 1,4-dicarbonyl compounds, and achieve the effect of low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A multi-substituted furan compound, the preparation method is as follows:
[0026] Add 50-80mL of chlorobenzene into a 250mL single-necked flask, then add 10mmol of tert-butyraldehyde and 10mmol of Reflux at 100°C for 12 hours, remove the solvent under reduced pressure to obtain a crude product, and then separate the product by flash column chromatography 41.6 mg (81%). The structure of the product characterizes the physical constants: 1 H NMR (400MHz, CDCl 3 )δ7.42-7.34(m,2H),7.31-7.27(m,1H),7.21-7.18(m,2H),6.53(d,J=3.4Hz,1H),5.85(d,J=3.4Hz ,1H),3.41(s,3H),0.97(s,9H); 13 C NMR (100MHz, CDCl 3 )δ166.57, 159.66, 145.92, 144.91, 129.43, 127.27, 127.19, 117.85, 103.84, 38.61, 32.70, 28.55.
Embodiment 2
[0028] Add 50-80mL of chlorobenzene into a 250mL single-necked flask, then add 10mmol of tert-butyraldehyde and 10mmol of Reflux at 100°C for 12 hours, remove the solvent under reduced pressure to obtain a crude product, and then separate the product by flash column chromatography 28.8 mg (74%). The structure of the product characterizes the physical constants: 1 H NMR (400MHz, CDCl 3 )δ6.93(d, J=3.4Hz, 1H), 6.06(d, J=3.4Hz, 1H), 3.19(br, 6H), 1.30(s, 9H); 13 C NMR (100MHz, CDCl 3 )δ166.12, 160.71, 146.55, 117.33, 104.09, 32.98, 29.04.
Embodiment 3
[0030] Add 50-80mL of chlorobenzene into a 250mL single-necked flask, then add 10mmol of tert-butyraldehyde and 10mmol of Reflux at 100°C for 12 hours, remove the solvent under reduced pressure to obtain a crude product, and then separate the product by flash column chromatography 33.4 mg (75%). The structure of the product characterizes the physical constants: 1 H NMR (400MHz, CDCl 3 )δ6.97(d, J=3.4Hz, 1H), 6.05(d, J=3.4Hz, 1H), 3.56(br, 4H), 1.30(s, 9H), 1.25(br, 6H); 13 C NMR (100MHz, CDCl 3 )δ165.84, 159.80, 147.09, 117.34, 104.15, 32.94, 29.05.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com