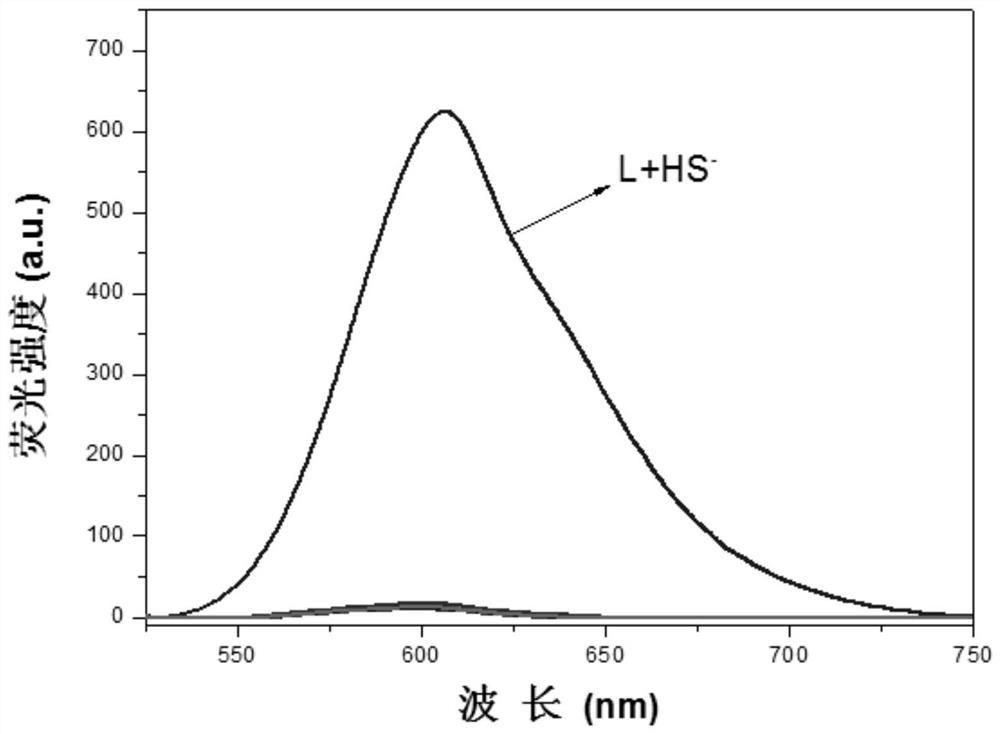
A kind of H2S fluorescent probe based on 4-styrene pyridinium salt long-wave emission recognition and its synthesis method and application
A fluorescent probe and probe technology, applied in luminescent materials, fluorescence/phosphorescence, chemical instruments and methods, etc., can solve the problems of long response time, large radiation energy, short emission wavelength, etc., to achieve easy separation and purification, good resistance Interference ability, effect of short response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) The specific synthetic steps of compound 1 are as follows:
[0030]
[0031] 4-Diethylamino salicylaldehyde (1.54g, 8.0mmol), 4-picoline salt (1.88g, 8.0mmol) and piperidine (0.8mmol) were dissolved in ethanol, heated to reflux for 12h, and the solvent was spun off. The crude product was purified by thin-layer column chromatography using CH 3 OH:CH 2 Cl 2 =1:50 (v / v) was used as eluent, and 2.05g of compound 1 was isolated with a yield of 62.5%;
[0032] 1 H NMR (400MHz, DMSO-d 6 )δ10.13(s,1H),8.59(d,J=6.5Hz,2H),8.03-7.93(m,3H), 7.49(d,J=9.0Hz,1H),7.15(d,J=16.0 Hz,1H),6.33(dd,J=9.0,2.4Hz,1H),6.21(d,J=2.4Hz,1H),4.15(s,3H),3.39(q,J=7.0Hz,4H), 1.15(t,J=7.0Hz,6H).
[0033] (2) The specific synthesis steps of fluorescent probe L are as follows:
[0034]
[0035] Compound 1 (410mg, 1mmol), 2,4-dinitrofluorobenzene (223mg, 1.2mmol), potassium carbonate (207mg, 1.5mmol), were dissolved in 10mL DMF, and reacted at room temperature for 6h. After the reaction, was...
Embodiment 2
[0039] (1) Synthesis of Compound 1
[0040] 4-Diethylamino salicylaldehyde (1.54g, 8.0mmol), 4-picoline salt (3.76g, 16.0mmol) and piperidine 16mmol were dissolved in ethanol, heated to reflux for 8h, and the solvent was spun off. The crude product was purified by thin-layer column chromatography using CH 3 OH:CH 2 Cl 2 =1: 100 (v / v) was used as the eluent to obtain compound 1;
[0041] (2) Synthesis of fluorescent probe L
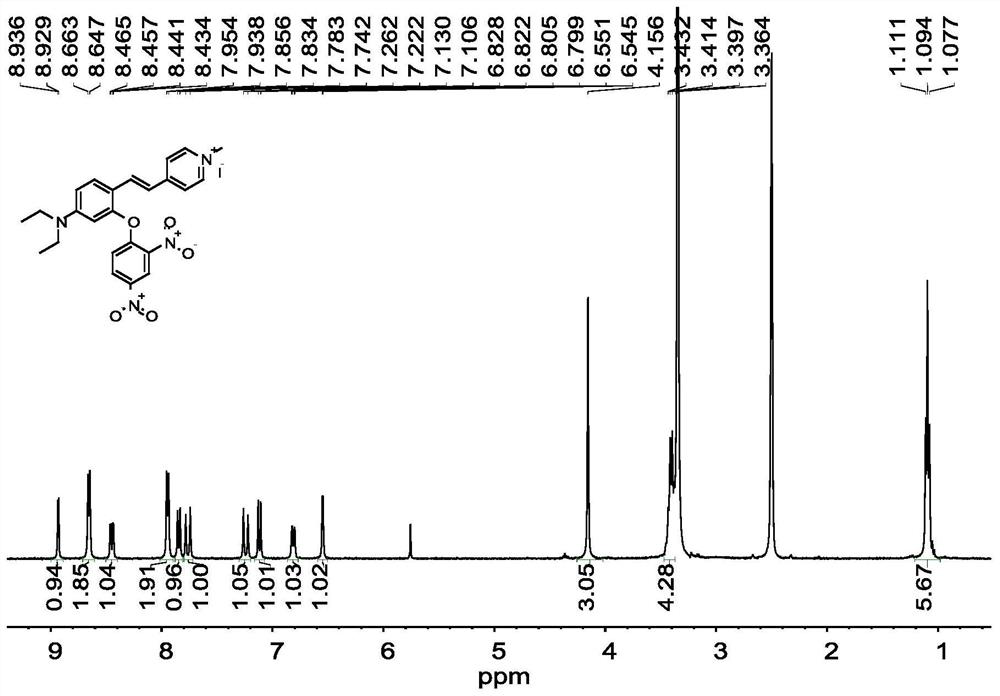
[0042] Compound 1 (410mg, 1.0mmol), 2,4-dinitrofluorobenzene (278.5mg, 1.5mmol), potassium carbonate (276mg, 2.0mmol) were dissolved in 15mL DMF, and reacted at room temperature for 8h. After the reaction, wash with water, extract with ethyl acetate, dry over anhydrous sodium sulfate, spin out the solvent, and purify the crude product by thin-layer column chromatography, using CH 3 OH:CH 2 Cl 2 =1: 80 (v / v) was used as the eluent to obtain 420 mg of probe L with a yield of 72.9%. fluorescent probe L 1 H NMR spectrum as figure 1 as shown, 13 C N...
Embodiment 3
[0044] (1) Synthesis of Compound 1
[0045] 4-Diethylamino salicylaldehyde (1.54g, 8.0mmol), 4-picoline salt (9.4g, 40.0mmol) and piperidine 8mmol were dissolved in ethanol, heated to reflux for 16h, and the solvent was spun off. The crude product was purified by thin-layer column chromatography using CH 3 OH:CH 2 Cl 2 =1: 100 (v / v) was used as the eluent to obtain compound 1;
[0046] (2) Synthesis of fluorescent probe L
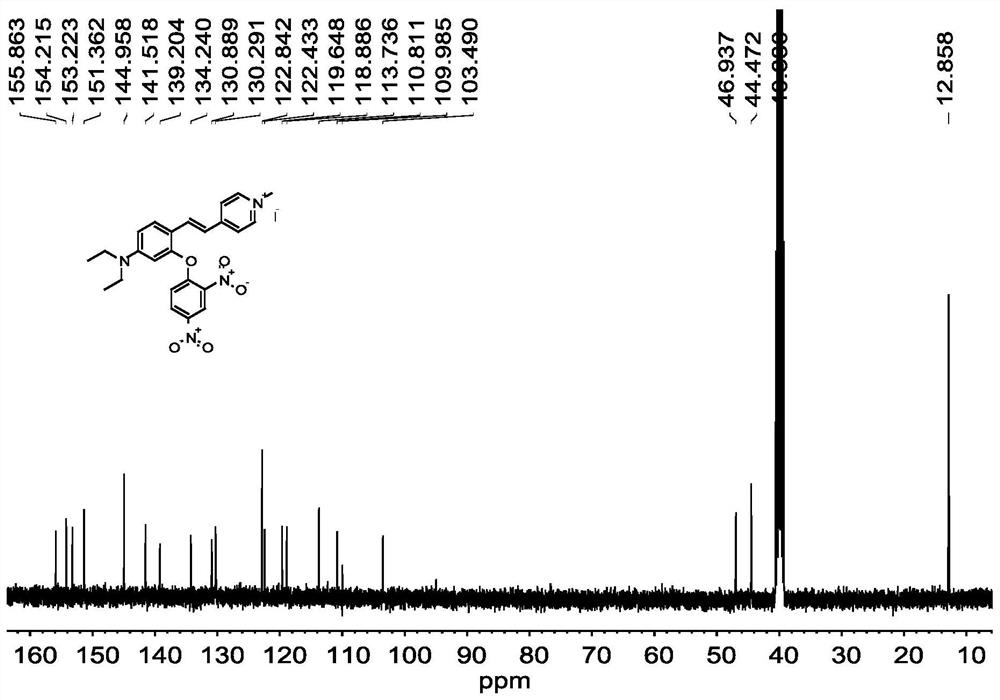
[0047] Compound 1 (410mg, 1.0mmol), 2,4-dinitrofluorobenzene (371mg, 2mmol), potassium carbonate (414mg, 3.0mmol) were dissolved in 15mL DMF, and reacted at room temperature for 10h. After the reaction, wash with water, extract with ethyl acetate, dry over anhydrous sodium sulfate, spin out the solvent, and purify the crude product by thin-layer column chromatography, using CH 3 OH:CH 2 Cl 2 =1:100 (v / v) was used as eluent to obtain fluorescent probe L. fluorescent probe L 1 H NMR spectrum as figure 1 as shown, 13 C NMR spectrum as figure 2 sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com