Amelioration and treatment of perinatal brain damage with pluripotent stem cells
A technology for pluripotent stem cells and brain damage, applied in the direction of non-embryonic pluripotent stem cells, artificially induced pluripotent cells, animal cells, etc., to achieve the effect of reducing brain damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0090] (2) Preparation and use of cell preparations and pharmaceutical compositions
[0091]The cell preparation and pharmaceutical composition of the present invention are not limited, and are obtained by suspending the Muse cells obtained in (1) above or a cell population containing Muse cells in physiological saline or an appropriate buffer (eg, phosphate-buffered saline). In this case, when the number of Muse cells isolated from autologous or allogeneic tissue is small, the cells are cultured and proliferated until a predetermined cell concentration is obtained before administration. In addition, as reported (International Publication No. WO2011 / 007900), Muse cells do not become tumorous, and cells recovered from living tissues contain cells in an undifferentiated state, which are less likely to become cancerous and safe. In addition, the culture of recovered Muse cells is not particularly limited, and can be performed in a normal growth medium (for example, α-minimum esse...
Embodiment 1
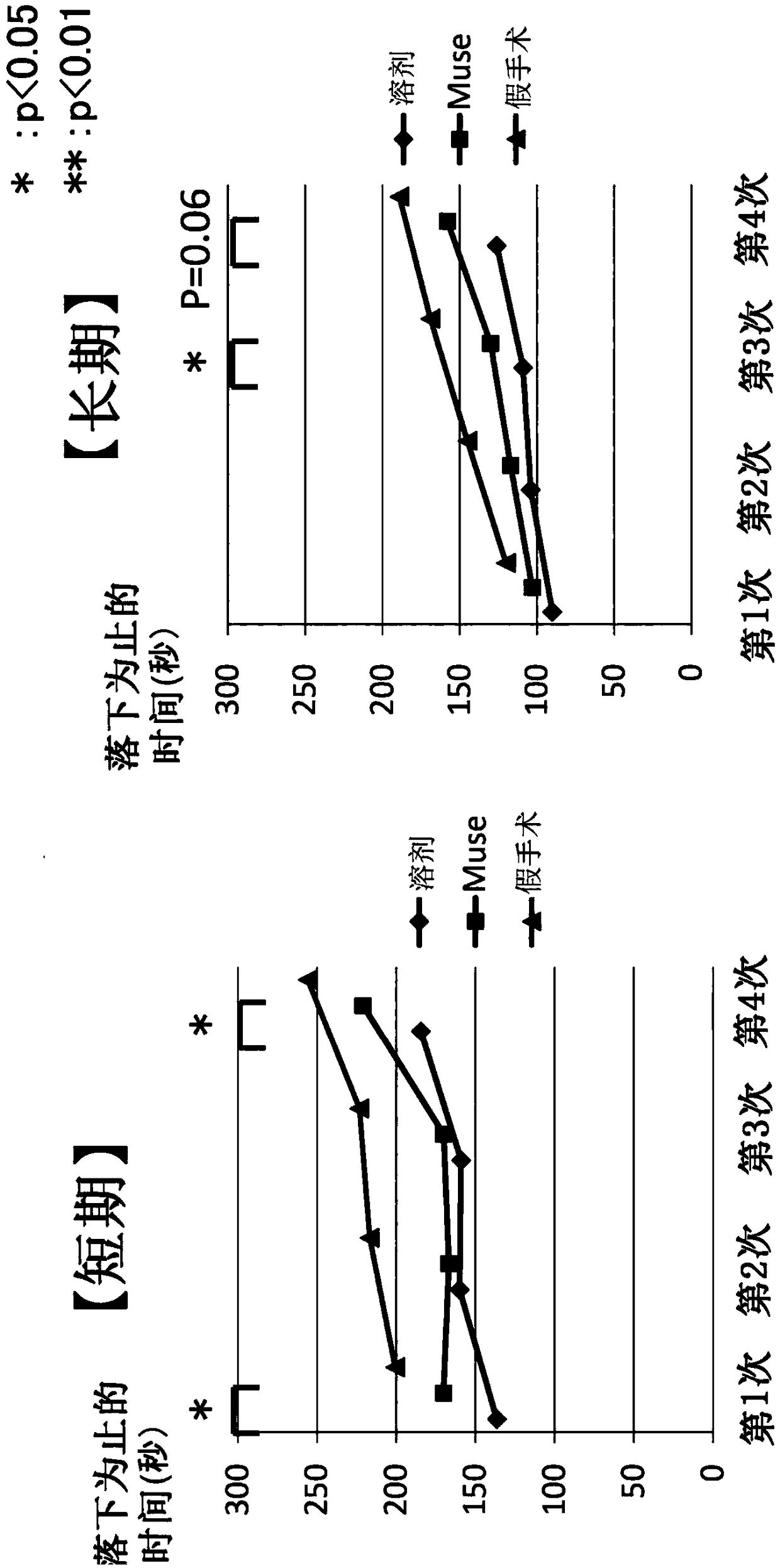
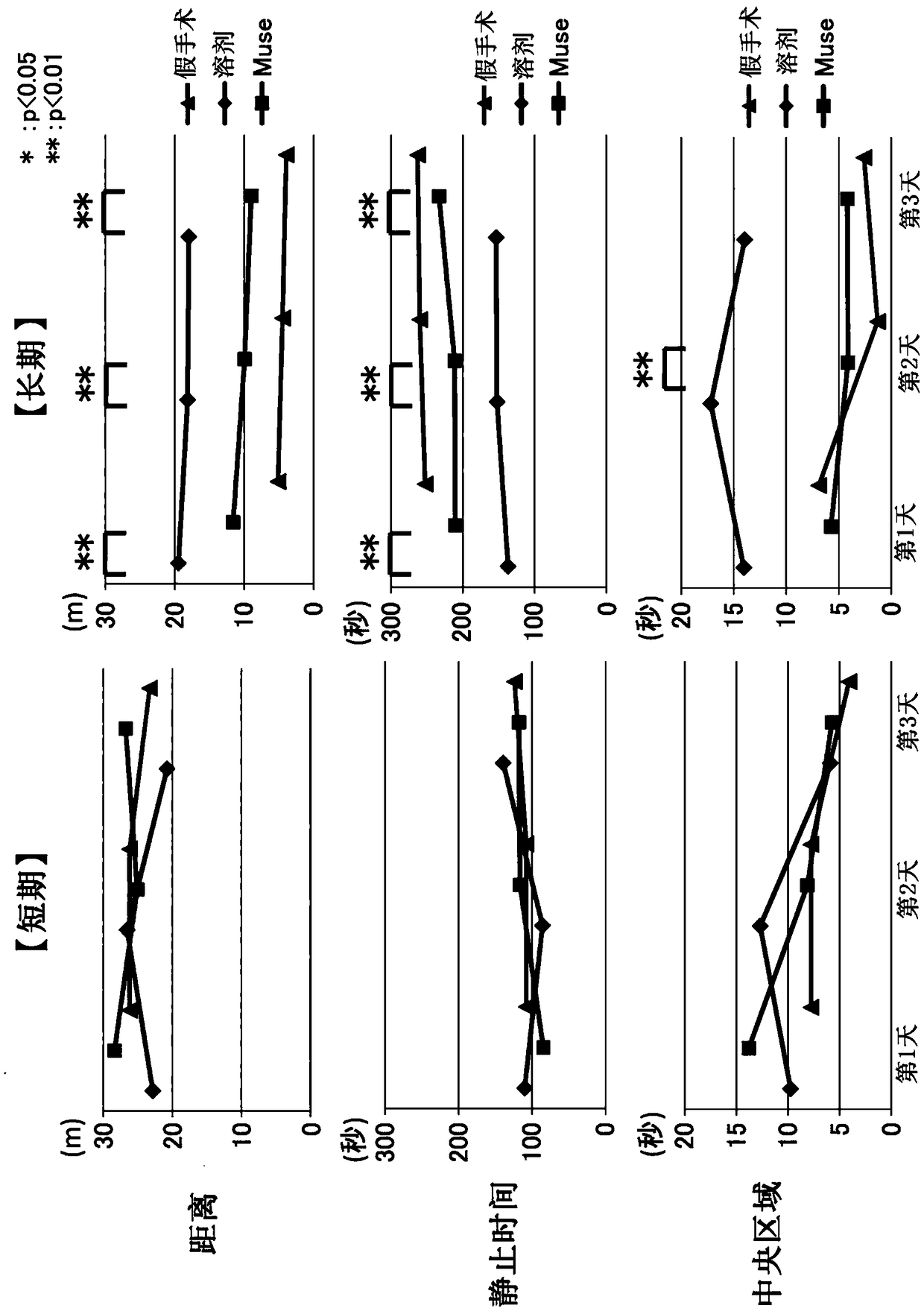
[0108] Embodiment 1: the preparation of perinatal hypoxic-ischemic encephalopathy (HIE) model rats and the administration of Muse cells
[0109] The experimental protocol regarding the experimental animals used in this study was approved by the Animal Experiment Committee of the Faculty of Medicine, Nagoya University. Wistar / ST rats (babies) were purchased from Japan SLC Co., Ltd. (Shizuoka Prefecture, Japan), put into a cage with free intake of bait and water, and raised under a 12-hour light-dark cycle. The animal room and experimental space were maintained at 23°C throughout.
[0110] HIE model rats were prepared using Wistar / ST rats (7 days after birth) by a method based on the method described in Rice et al. (Ann. Neurol., vol.9, 131-141 (1981). Each rat was inhaled Anesthesia was performed with isoflurane. However, the left carotid artery was double ligated, and the space between the ligations was excised. After resting for 1 hour beside the mother mouse, it was placed ...
Embodiment 2
[0111] Example 2: Preparation of Muse cells and confirmation of brain tissue implantation
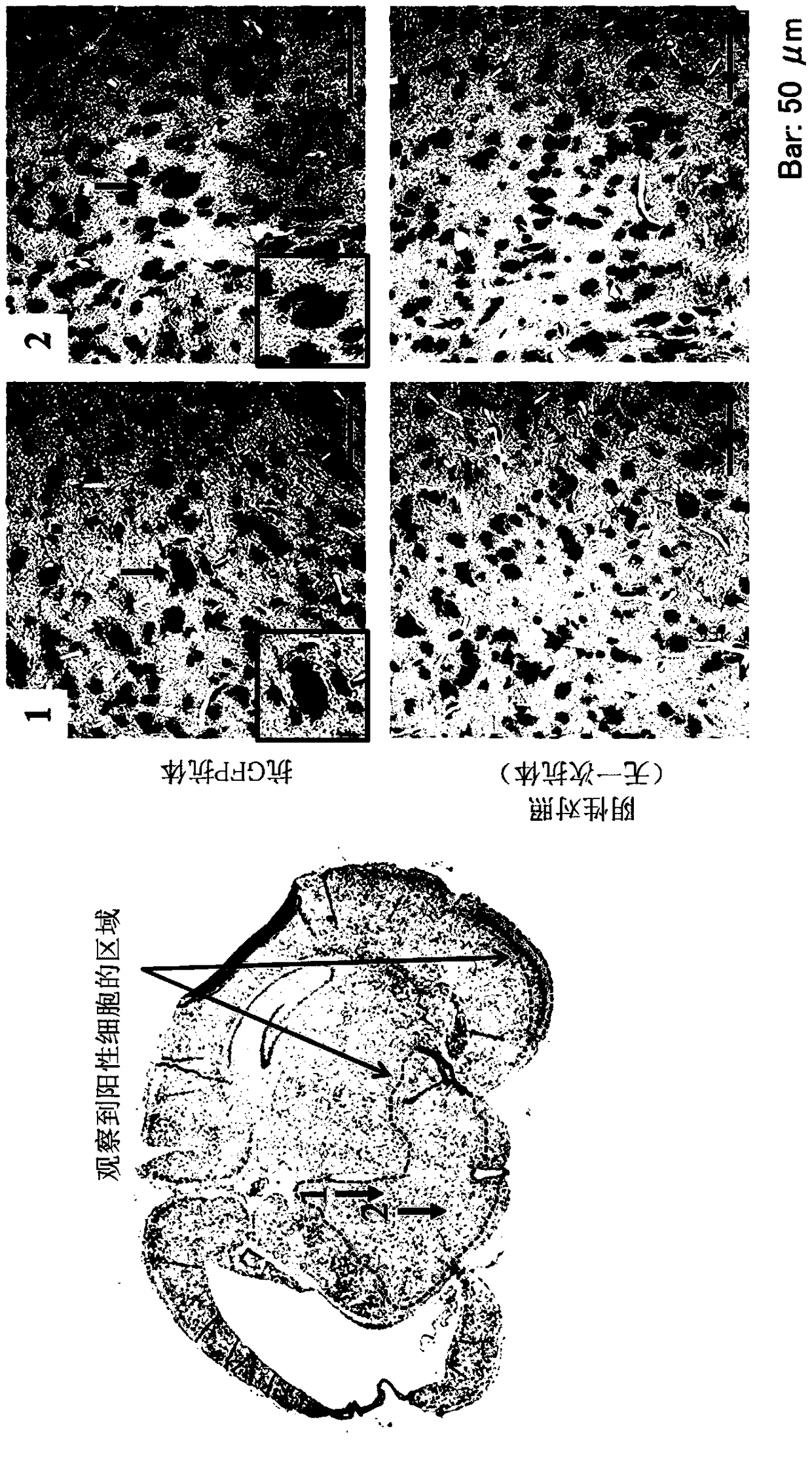
[0112] Muse cells were obtained according to the method described in International Publication No. WO2011 / 007900 on the isolation and identification of human Muse cells. The Muse cells used for transplantation were labeled with the expression of green fluorescent protein (GFP) in order to confirm implantation into the brain tissue, and the lentivirus-GFP gene was introduced into the Muse cells in advance. Muse cells labeled with GFP or cell populations containing Muse cells were isolated by FACS as double positive cells for GFP and SSEA-3. Then, as described in Example 1, Muse cells were administered.
[0113] Next, it was confirmed that Muse cells had implanted into brain tissue in the brains of 20-day-old rats administered with Muse cells. After the rats were euthanized, the brains were excised, and brain tissue slices were prepared according to a conventional method. The primary a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com