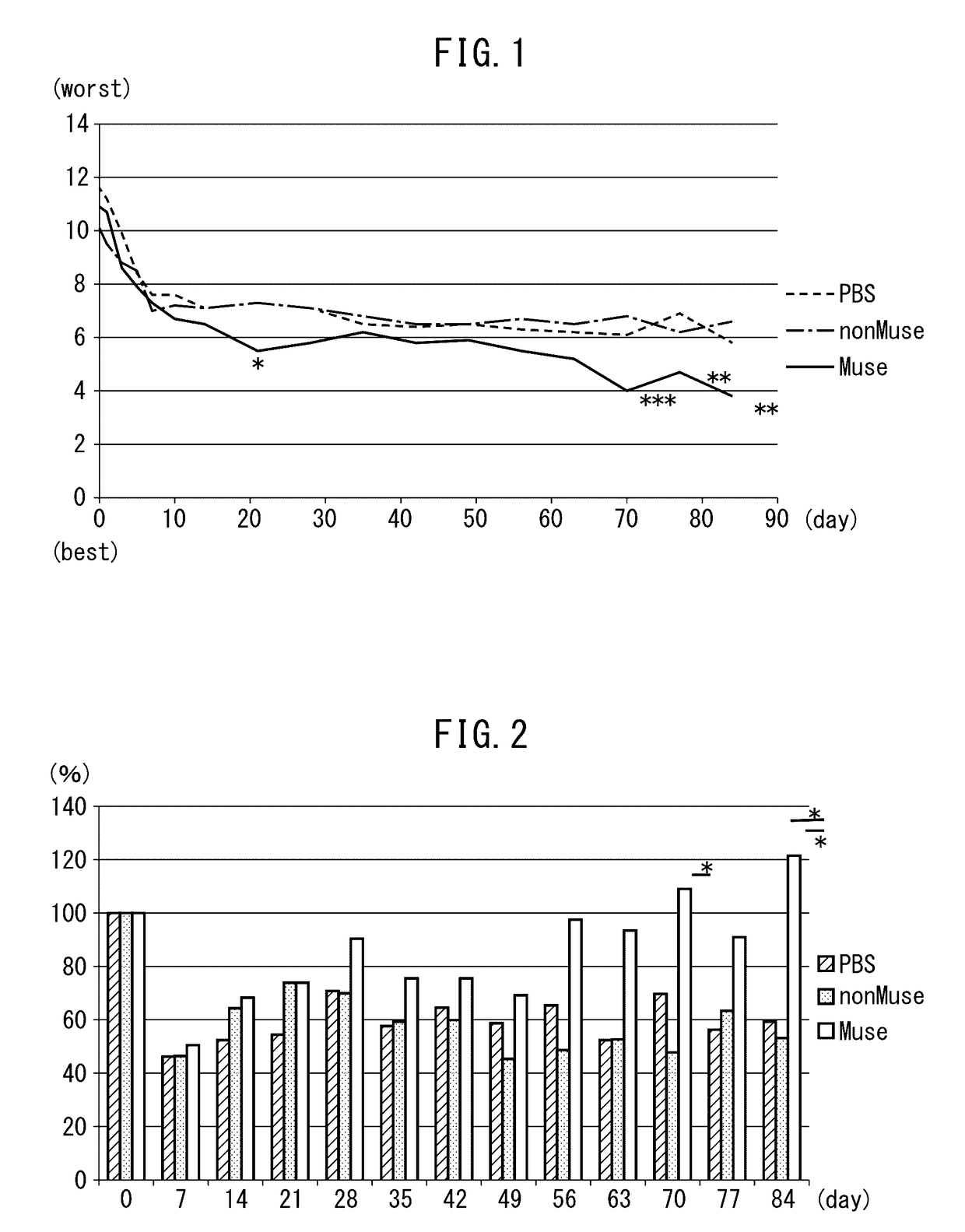
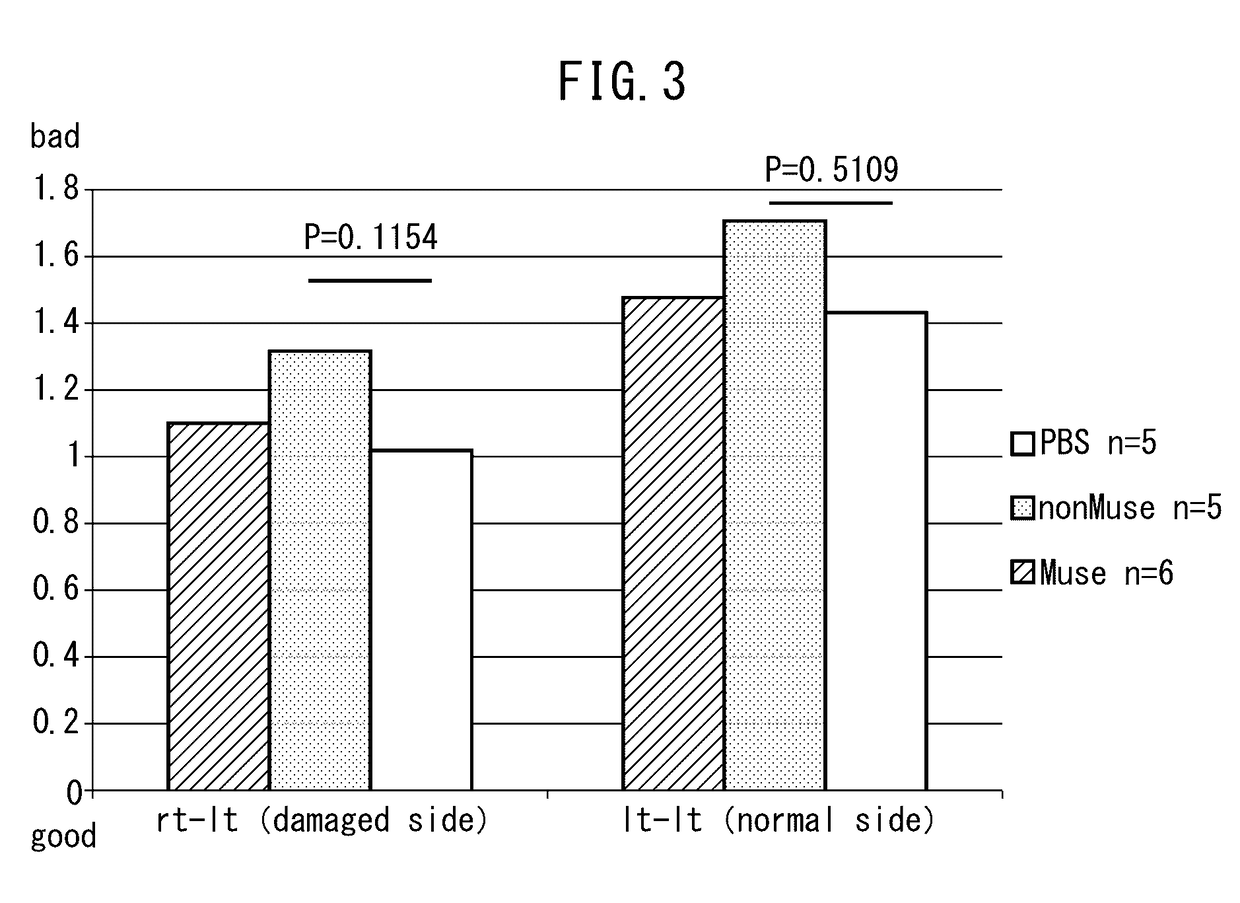
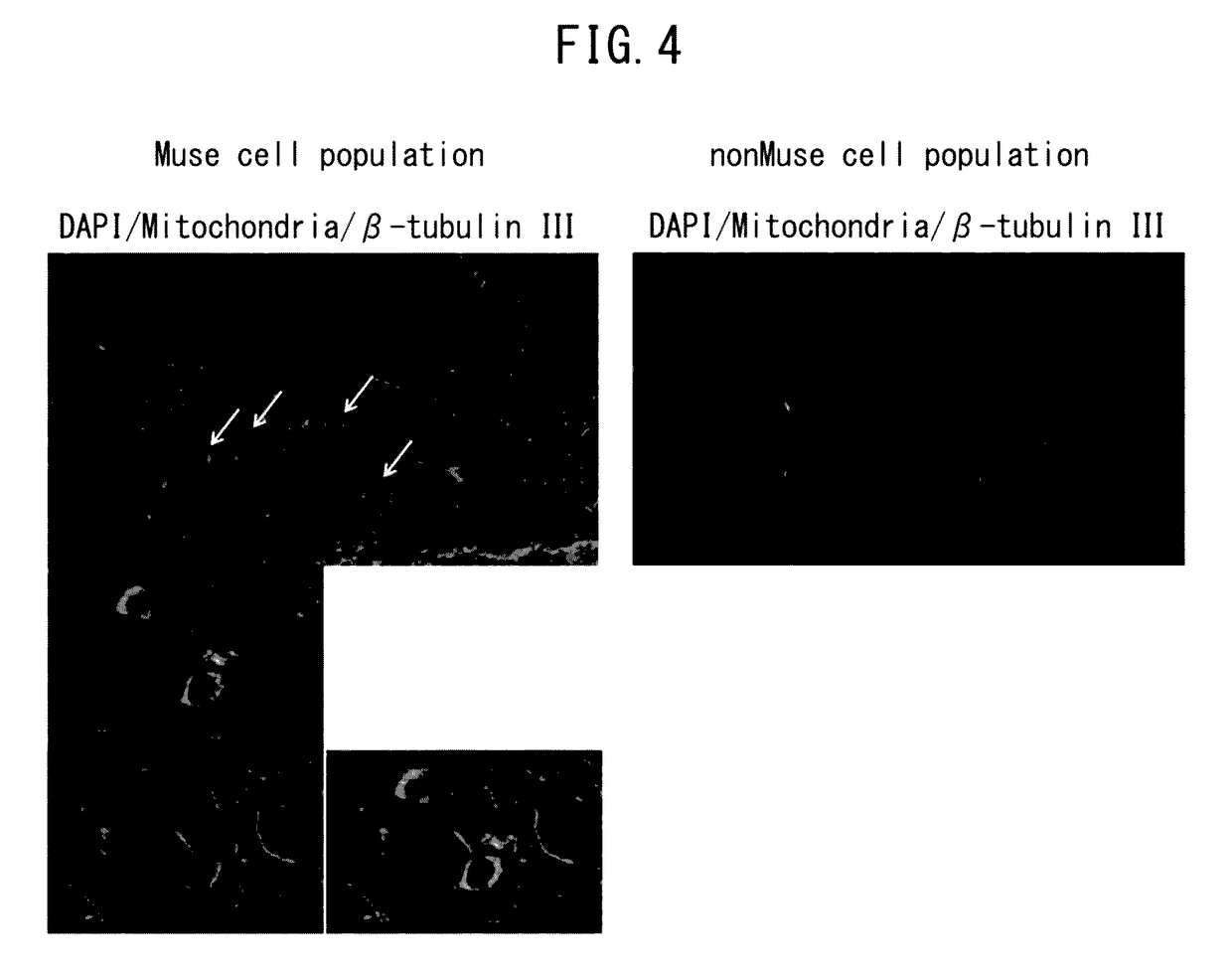
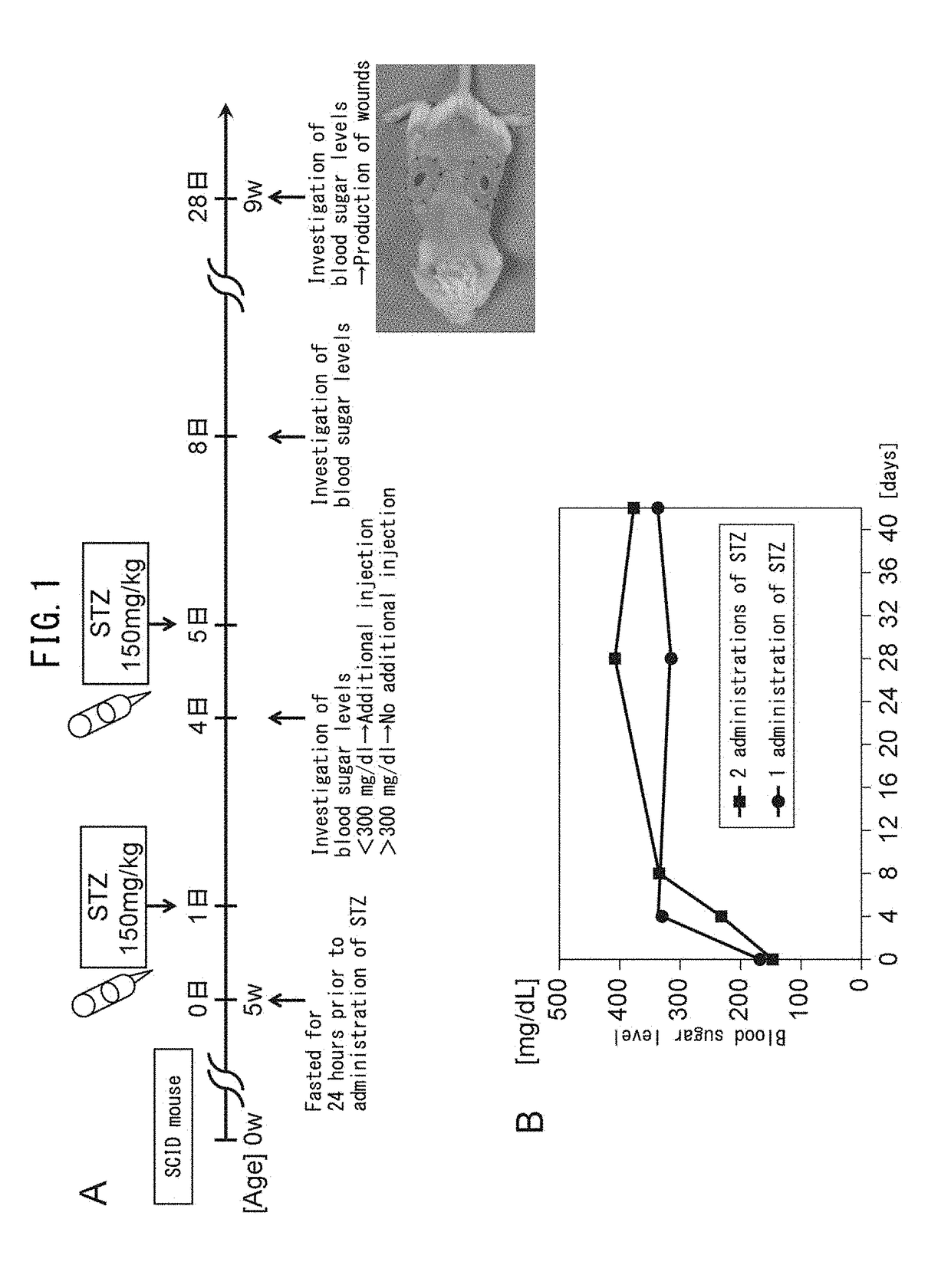
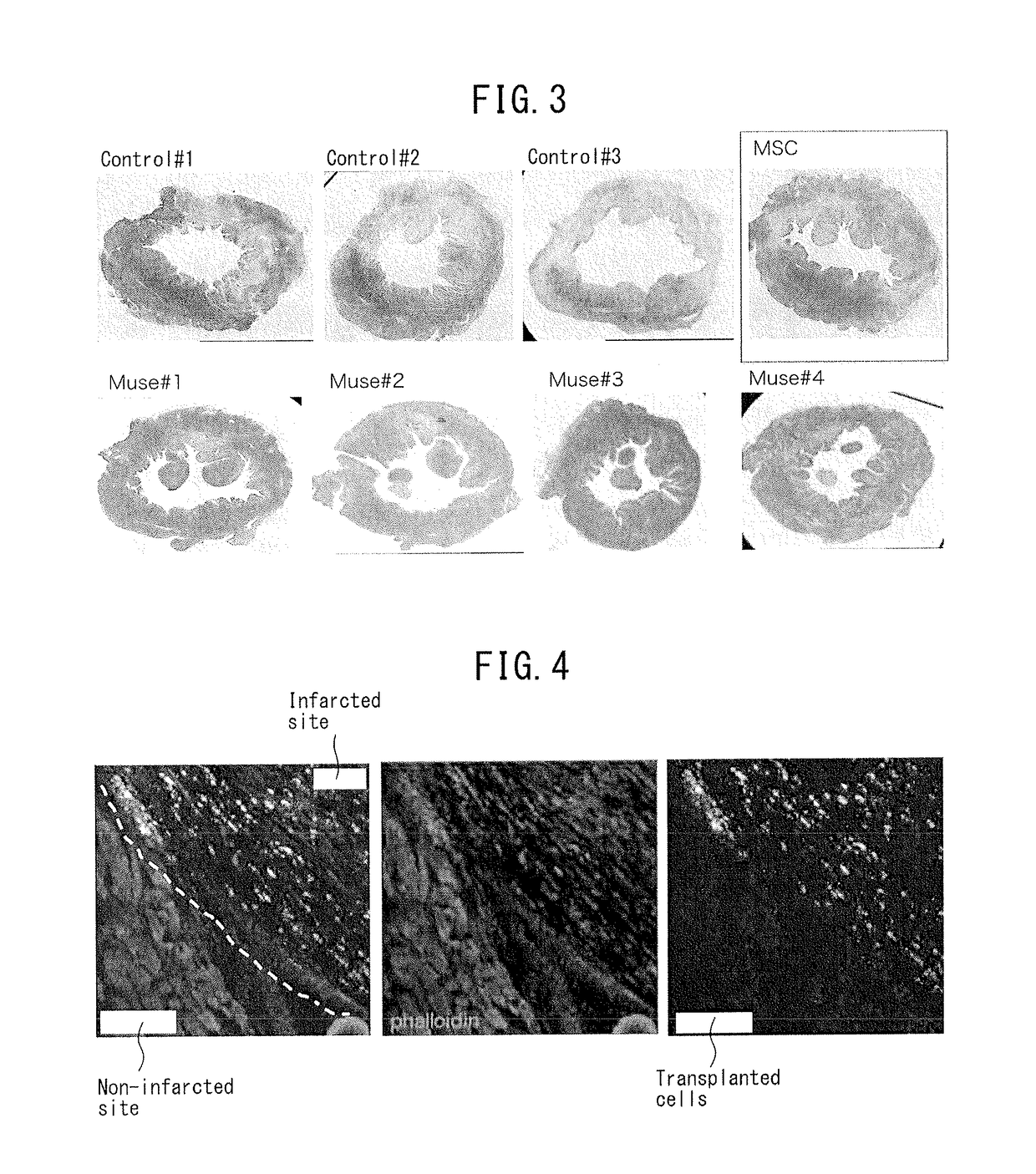
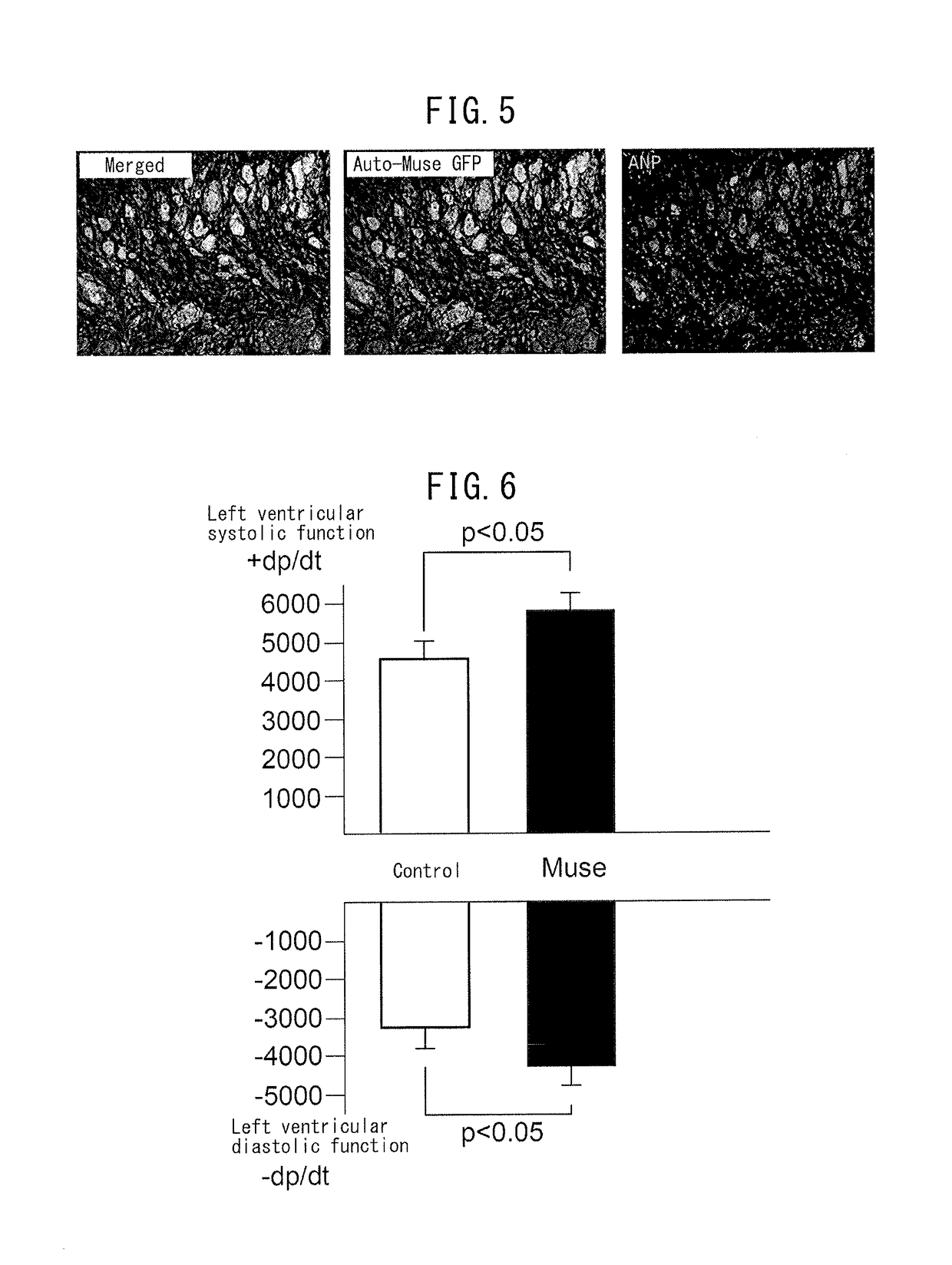
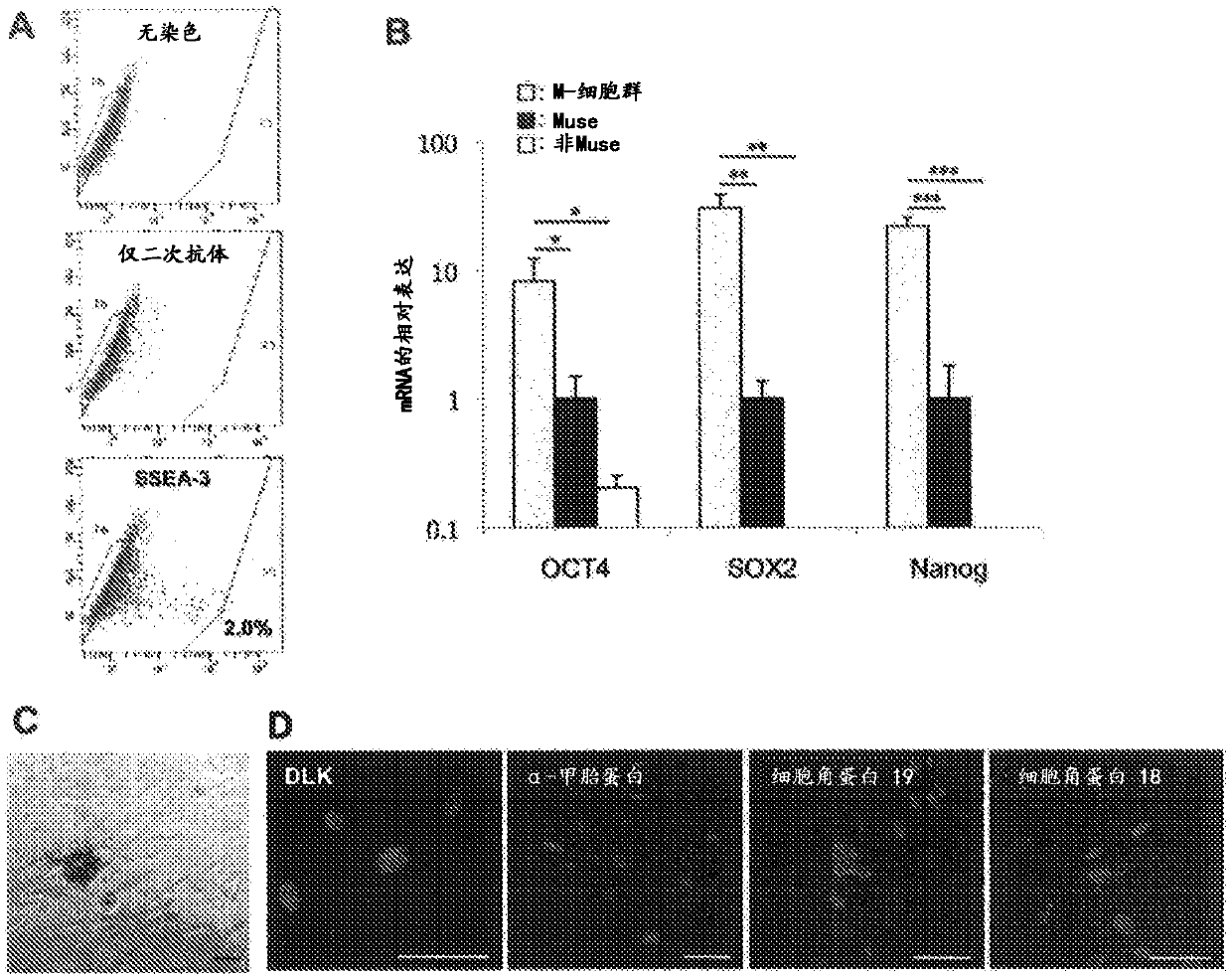
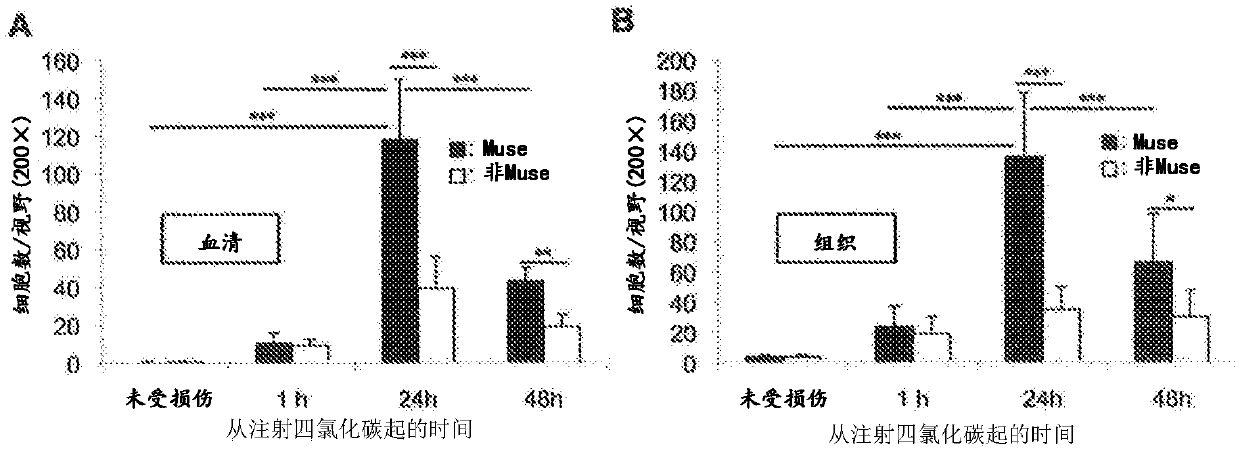
Patents
Literature
34 results about "Muse cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
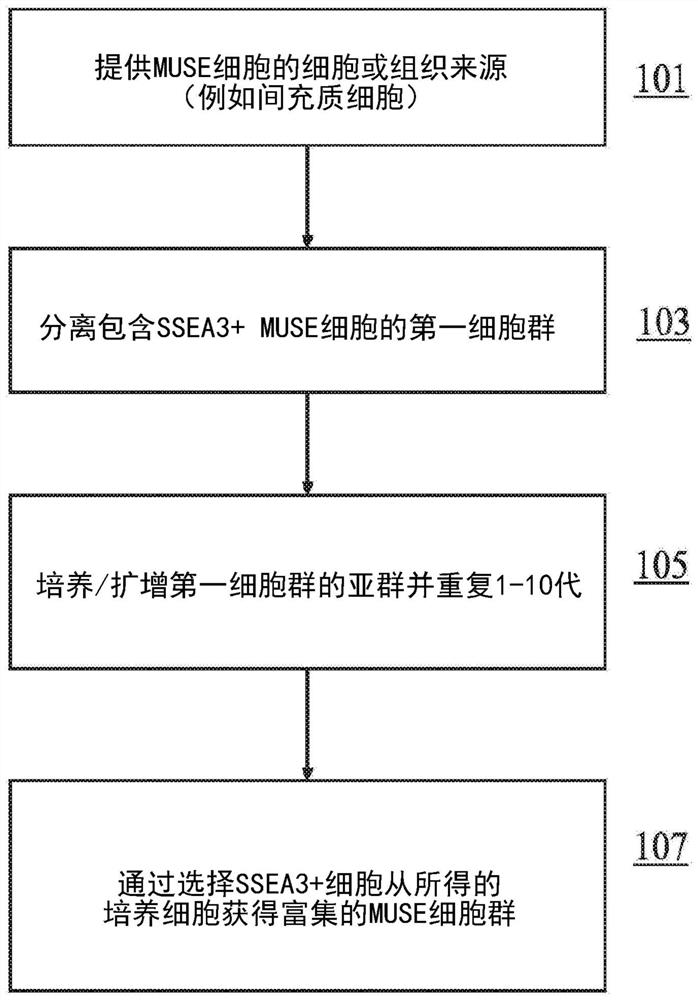
A Muse cell (Multi-lineage differentiating stress enduring cell) is an endogenous non-cancerous pluripotent stem cell. They reside in the connective tissue of nearly every organ, bone marrow and peripheral blood. They are collectable from commercially obtainable mesenchymal cells such as human fibroblasts, bone marrow-mesenchymal stem cells and adipose-derived stem cells. Muse cells are able to generate cells representative of all three germ layers from a single cell both spontaneously and under cytokine induction. Expression of pluripotency genes and triploblastic differentiation are self-renewable over generations. Muse cells do not undergo teratoma formation when transplanted into a host environment in vivo. This can be explained in part by their intrinsically low telomerase activity, eradicating the risk of tumorigenesis through unbridled cell proliferation. They were discovered in 2010 by Mari Dezawa and her research group. Clinical trials for acute myocardial infarction, stroke, epidermolysis bullosa and spinal cord injury are conducted by Life Science Institute, Inc., a group company of Mitsubishi Chemical Holdings company.
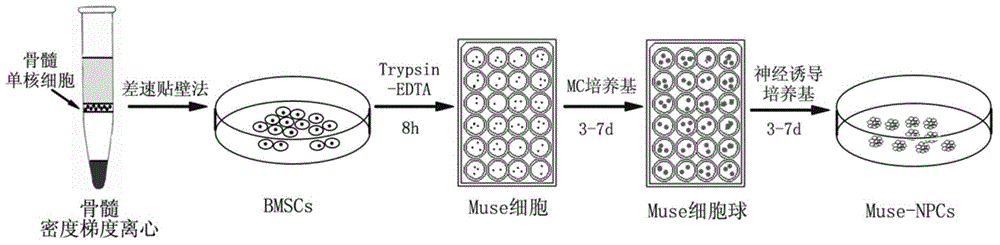
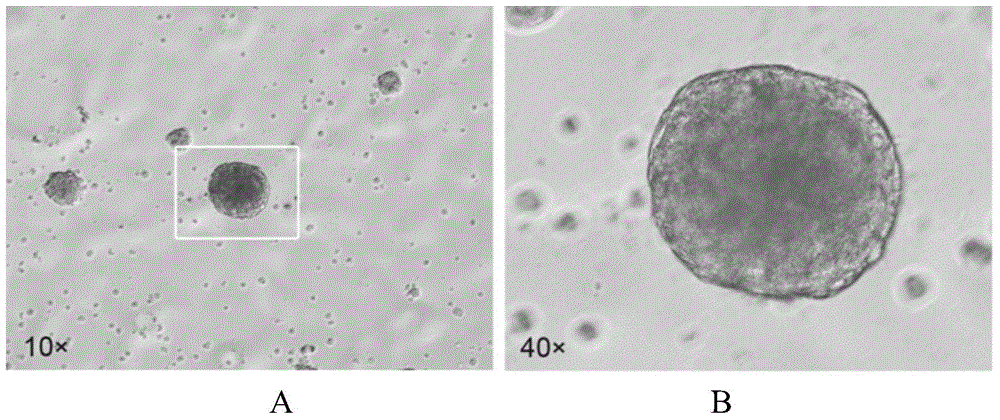
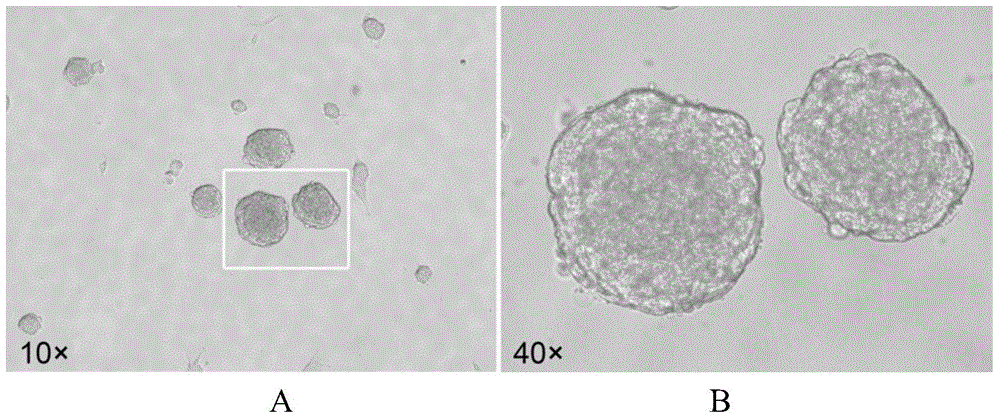
Method for inducing Muse cells in adult bone marrow into neural precursor cells (NPCs)
ActiveCN104946590AEasy accessPluripotent stem cell markerNervous system cellsCell phenotypePrecursor cell
The invention relates to the technical field of cytobiology, and provides a method for separating Muse cells from adult bone marrow and inducing into Muse-NPCs in vitro and also performs identification on the Muse cells and induced Muse-NPCs in the aspects of morphologic observation, cell phenotype, multiway differentiation capacity, tumorigenesis and the like. The related molecular markers for the Muse cells and Muse-NPCs cultured by the method are completely expressed. The invention also provides the Muse cells separated from adult bone marrow according to the method and the Muse-NPCs obtained by performing induced differentiation on the Muse cells. The Muse-NPCs are successfully induced to form in vitro, and can provide new favorable seed cells for cellular therapy of nerve injury and tissue engineering therapy in future by utilizing the characteristics of continuous proliferation, multiway differentiation and self derivation.
Owner:苏州美仑生物科技有限公司
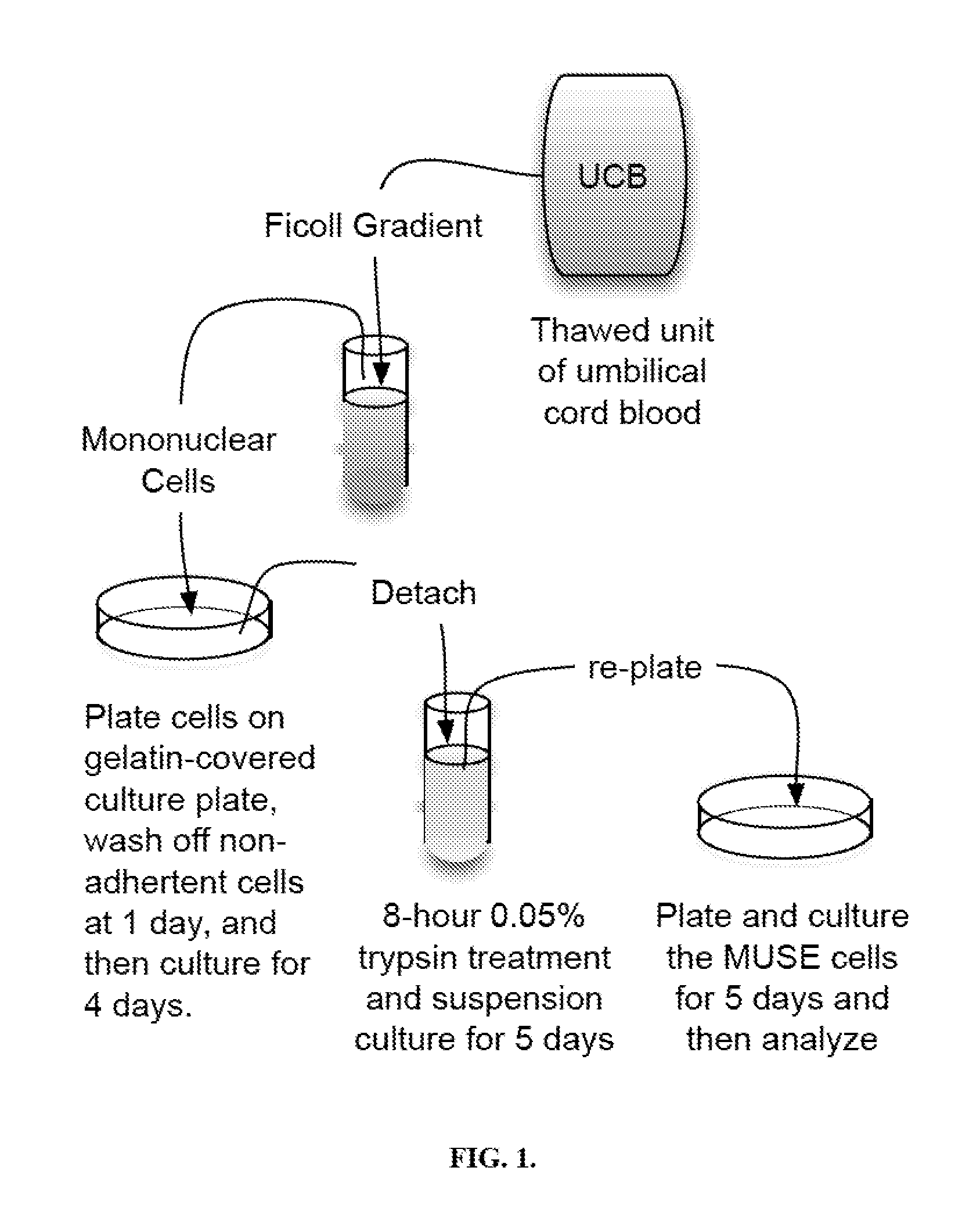
Muse cells isolation and expansion
Owner:RUTGERS THE STATE UNIV
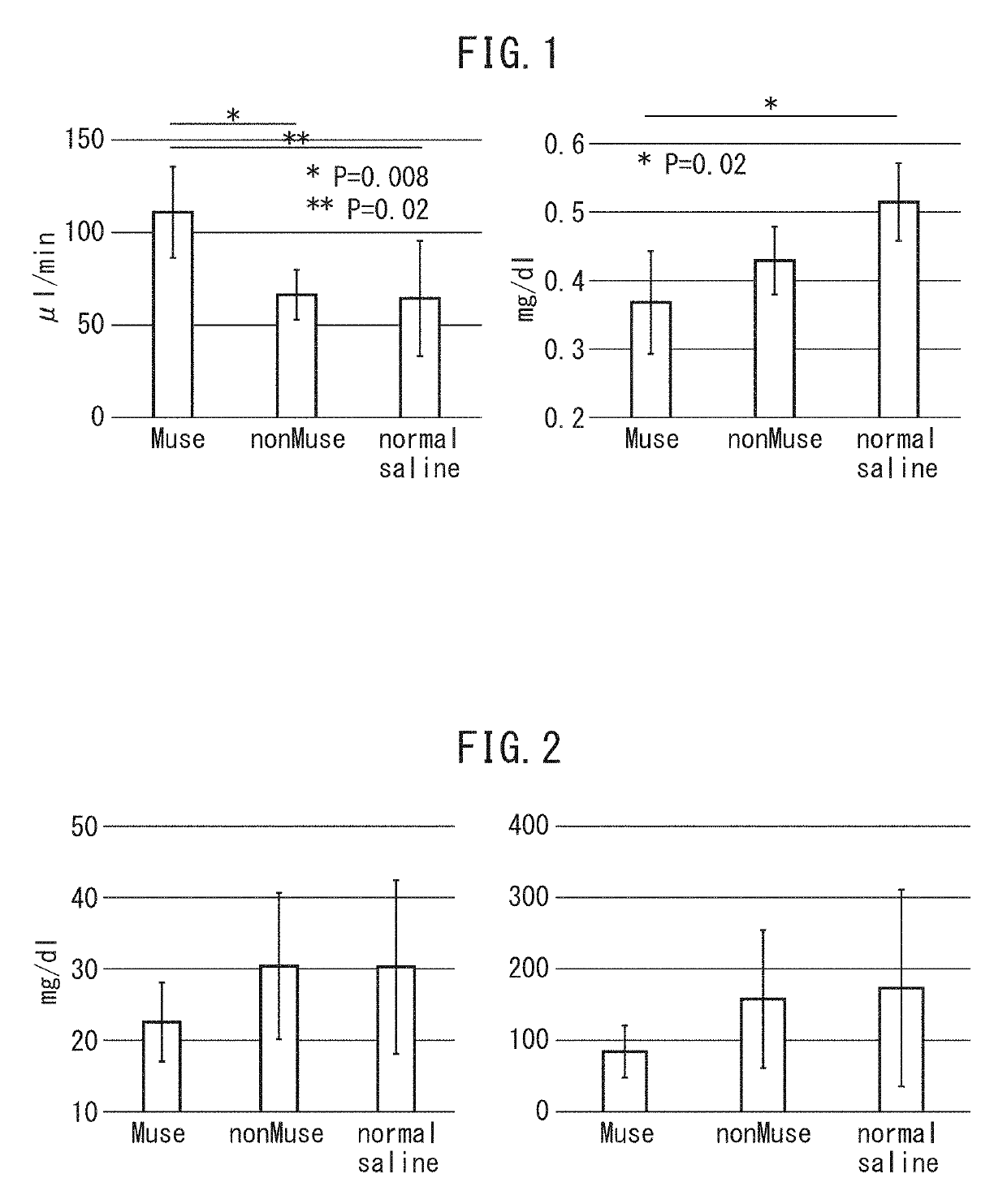
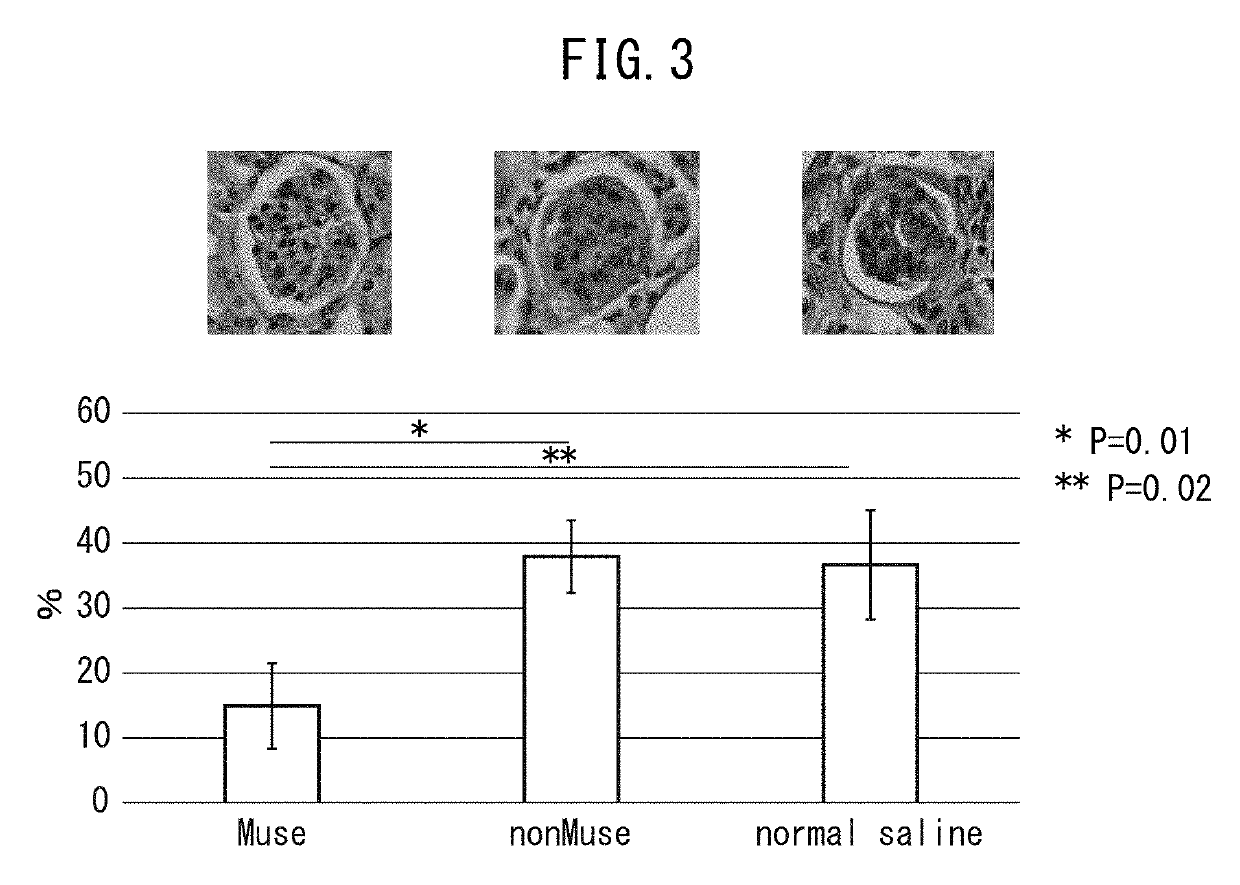
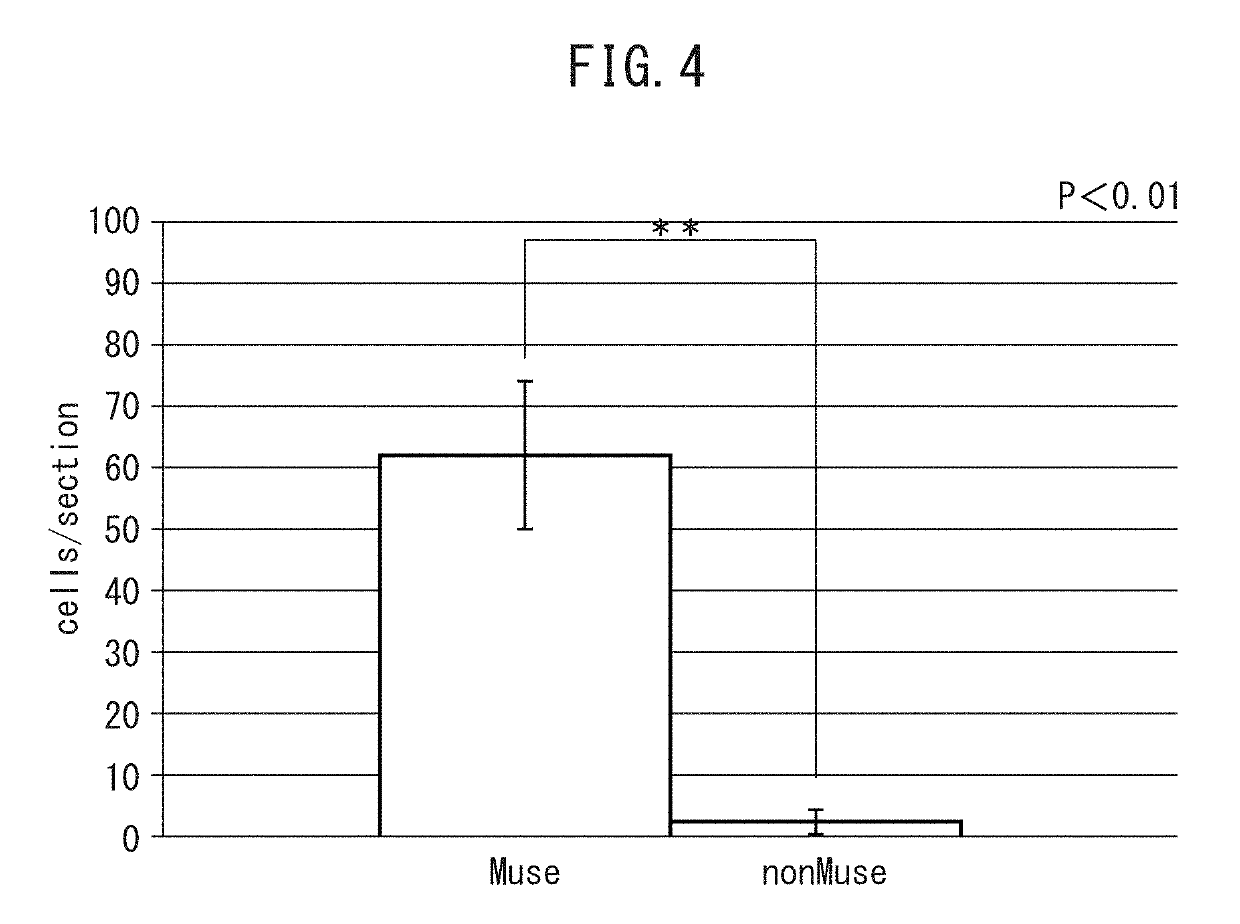
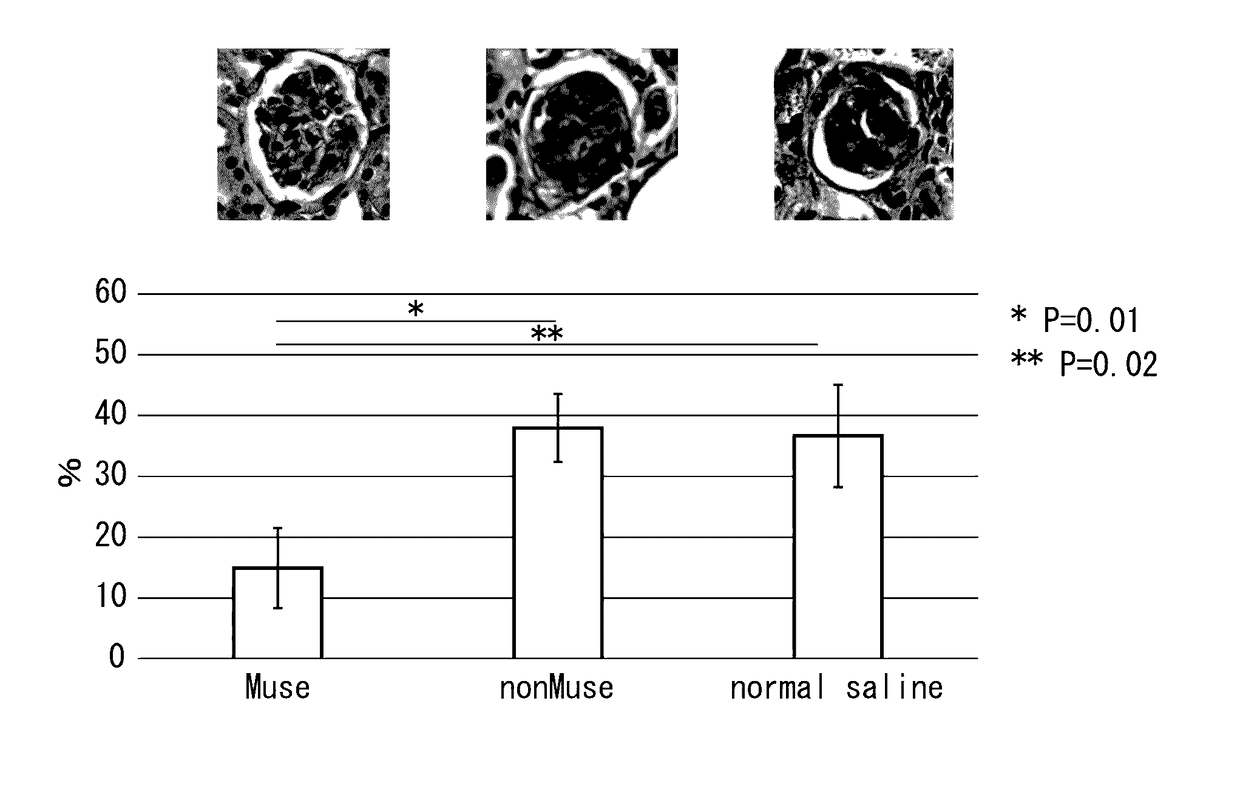
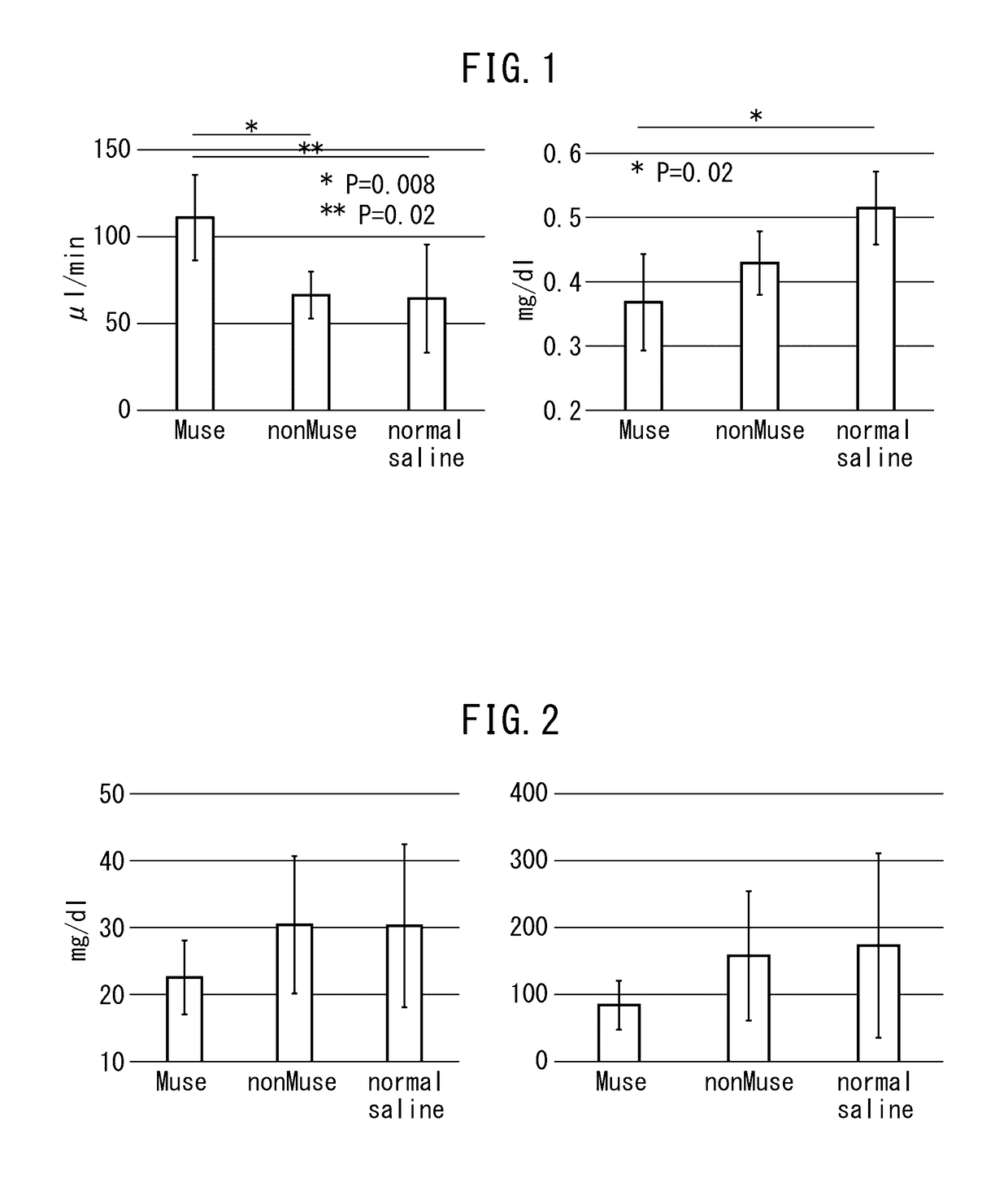
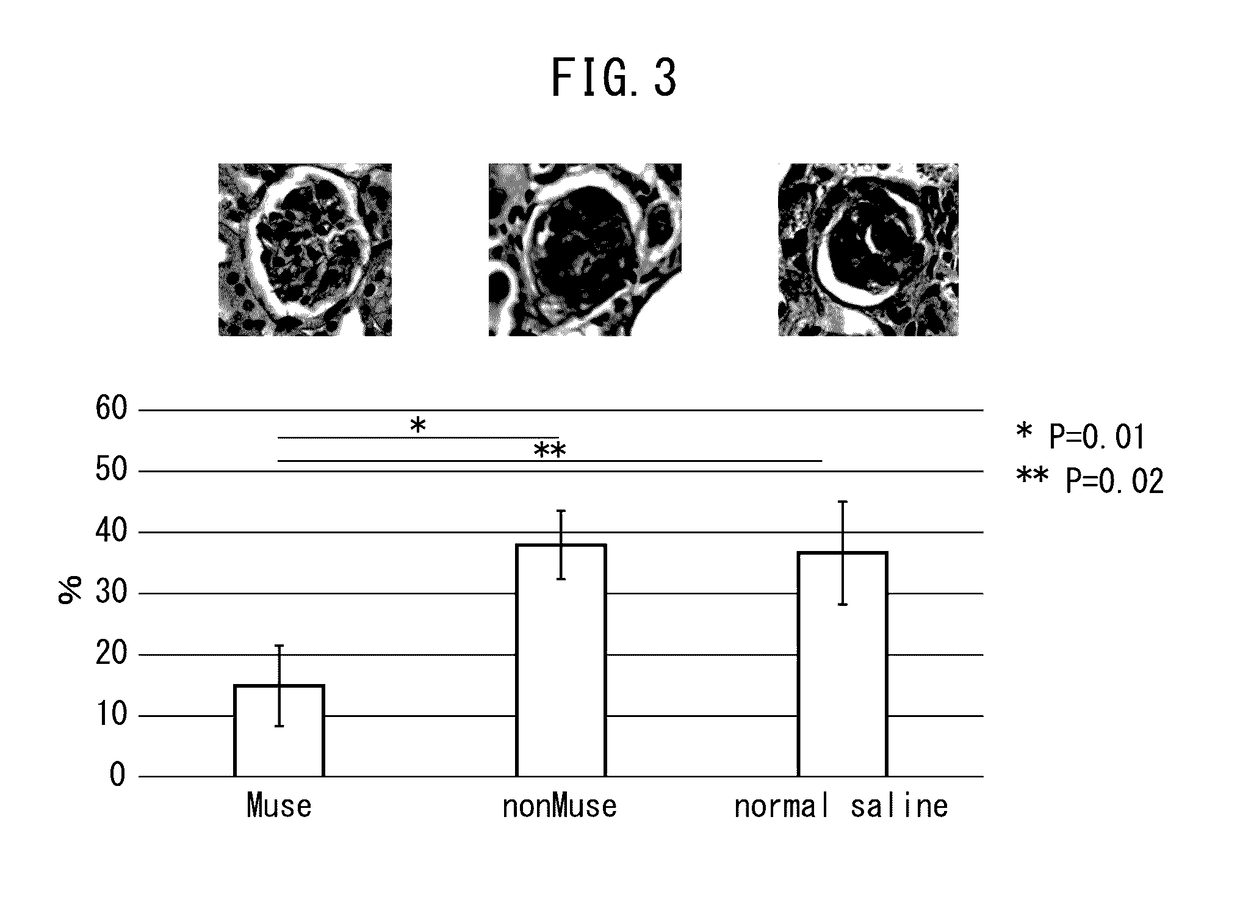
Pluripotent stem cell for treatment of chronic kidney disease
InactiveUS20160058800A1Inhibit progressImprove kidney functionUnknown materialsTissue cultureDiseaseVein
An object of the present invention is to provide a novel medical application to regenerative medicine that uses pluripotent stem cells (Muse cells). The present invention provides a cell preparation for treating chronic kidney disease that contains SSEA-3-positive pluripotent stem cells isolated from mesenchymal tissue in the body or cultured mesenchymal cells. The cell preparation of the present invention is based on a renal tissue regeneration mechanism by which Muse cells are made to selectively accumulate at a site of kidney disease and differentiate into cells that compose the kidney by administering Muse cells intravenously to a subject having the aforementioned disease.
Owner:CLIO +1
Pharmaceutical composition including migratory factor for guiding pluripotent stem cells to injury
ActiveUS20160008340A1Enhances chemotactic activityHigh activityOrganic chemistryAntipyreticBULK ACTIVE INGREDIENTActive ingredient
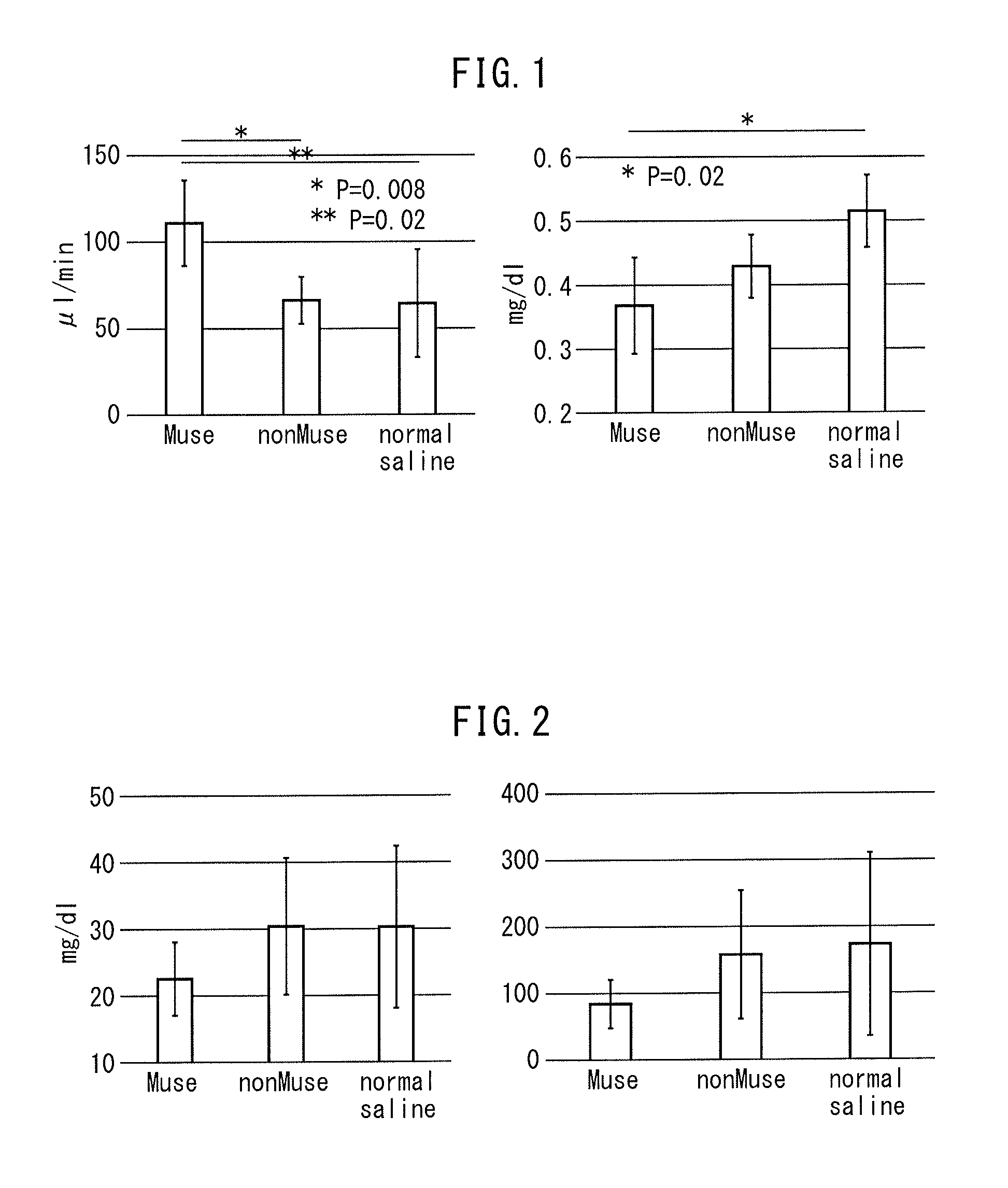
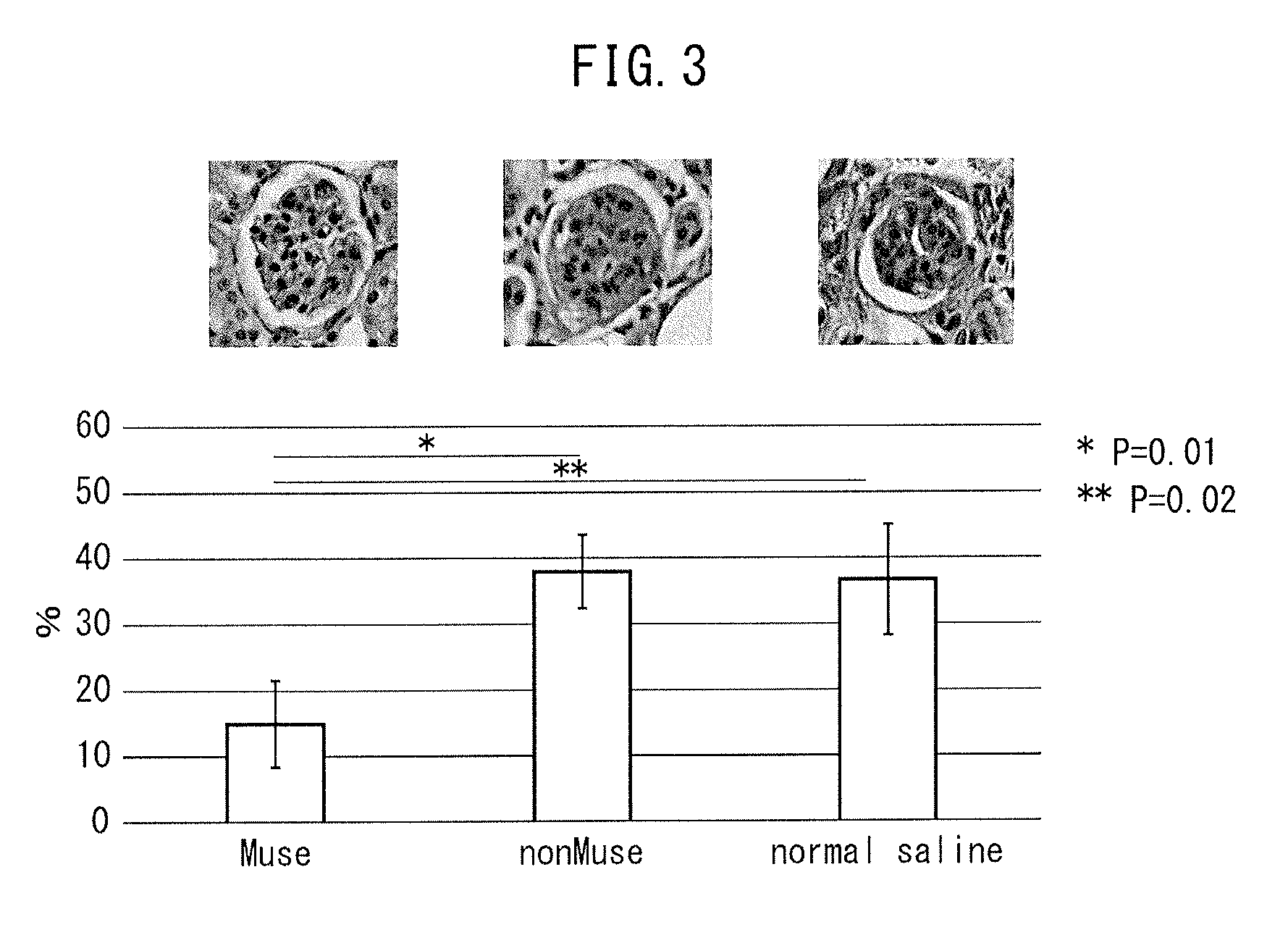
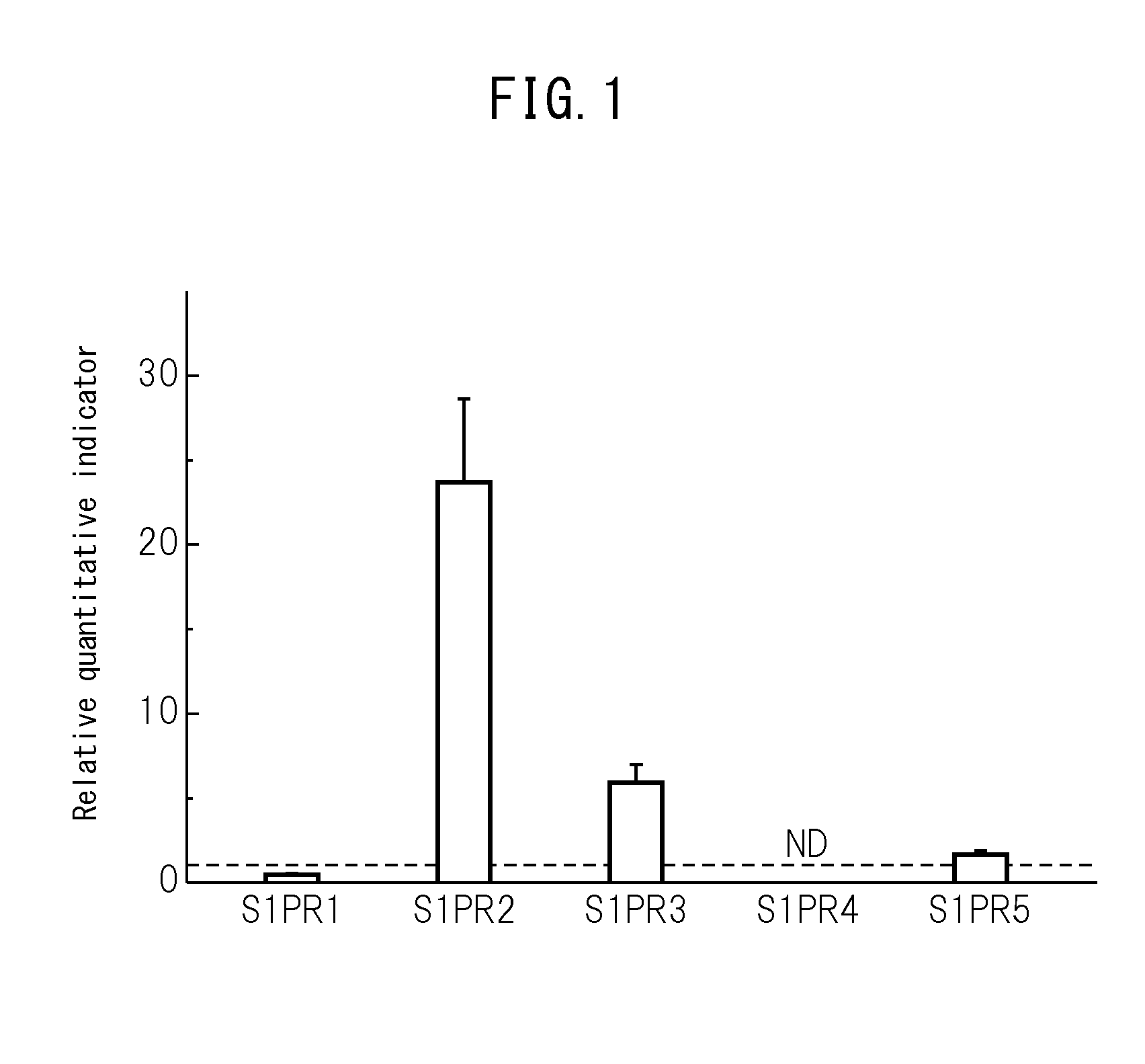
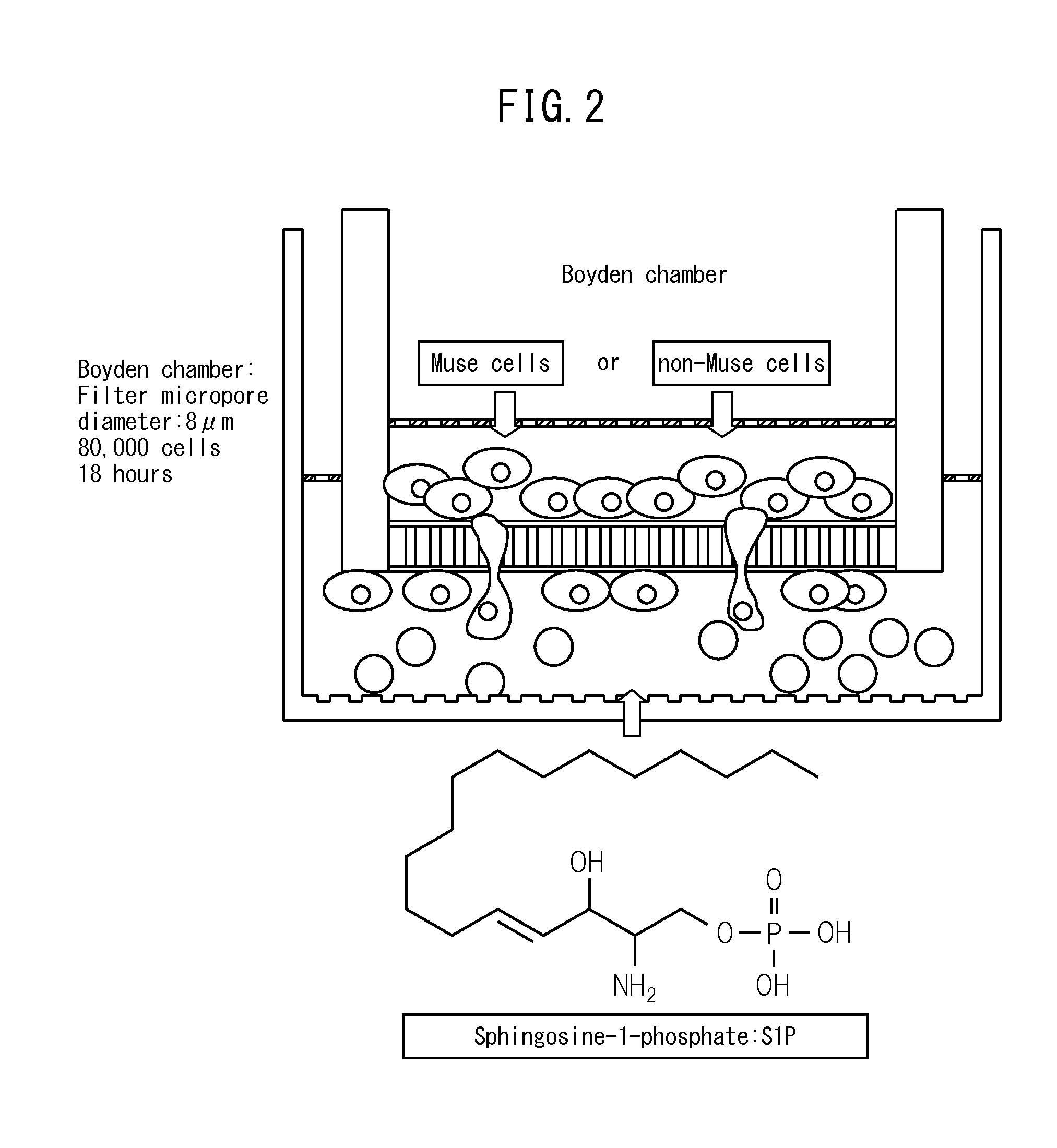
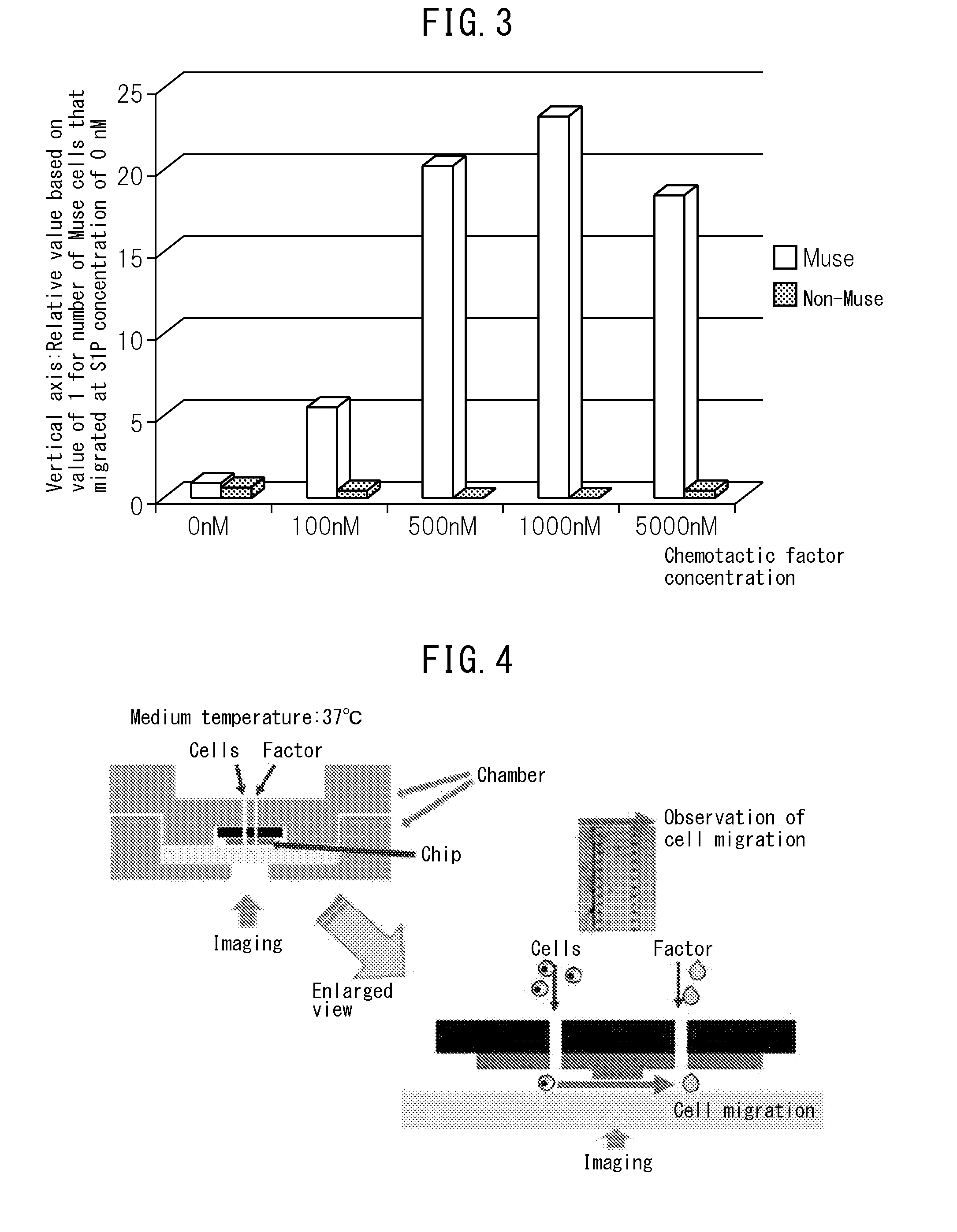
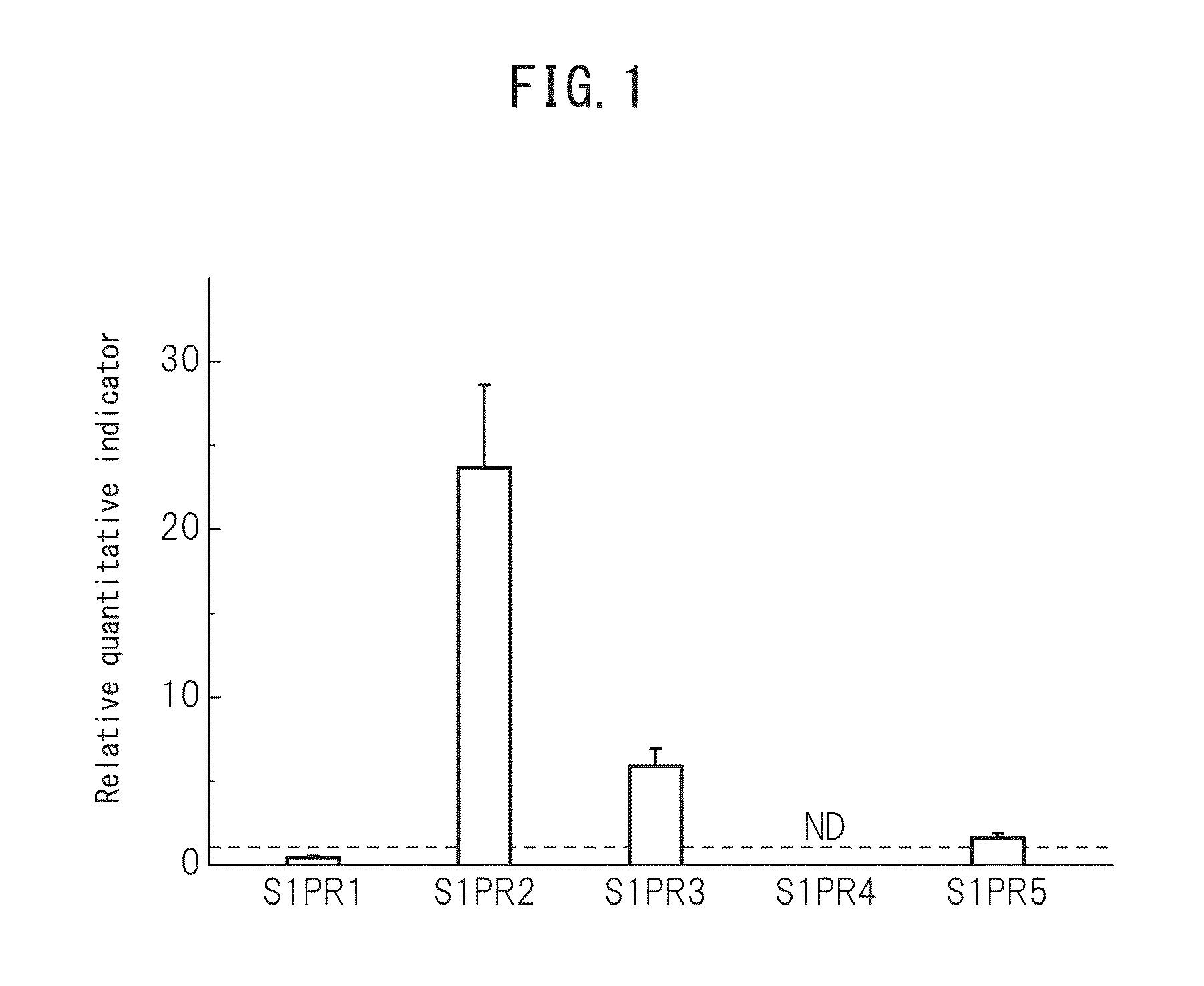
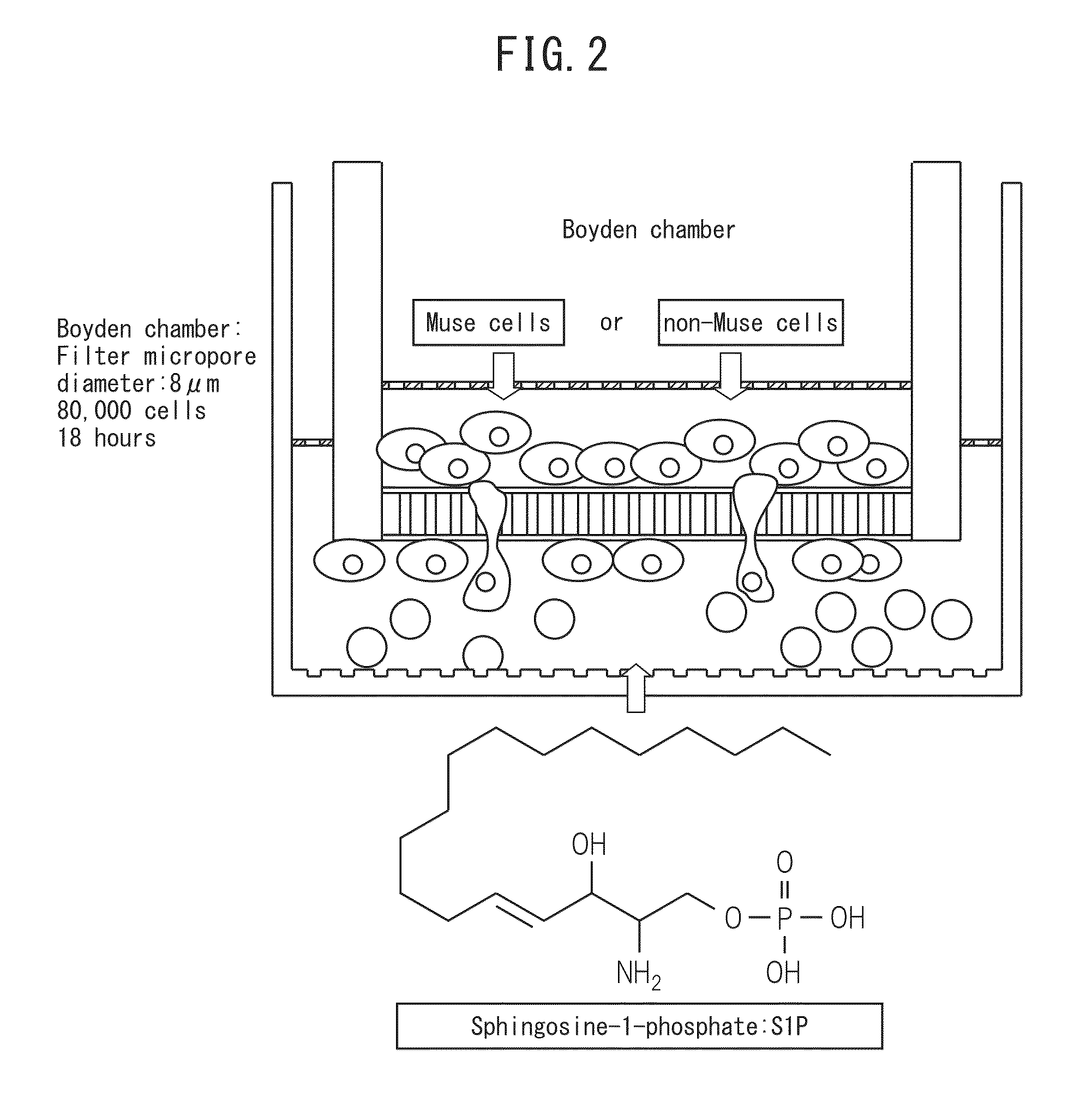
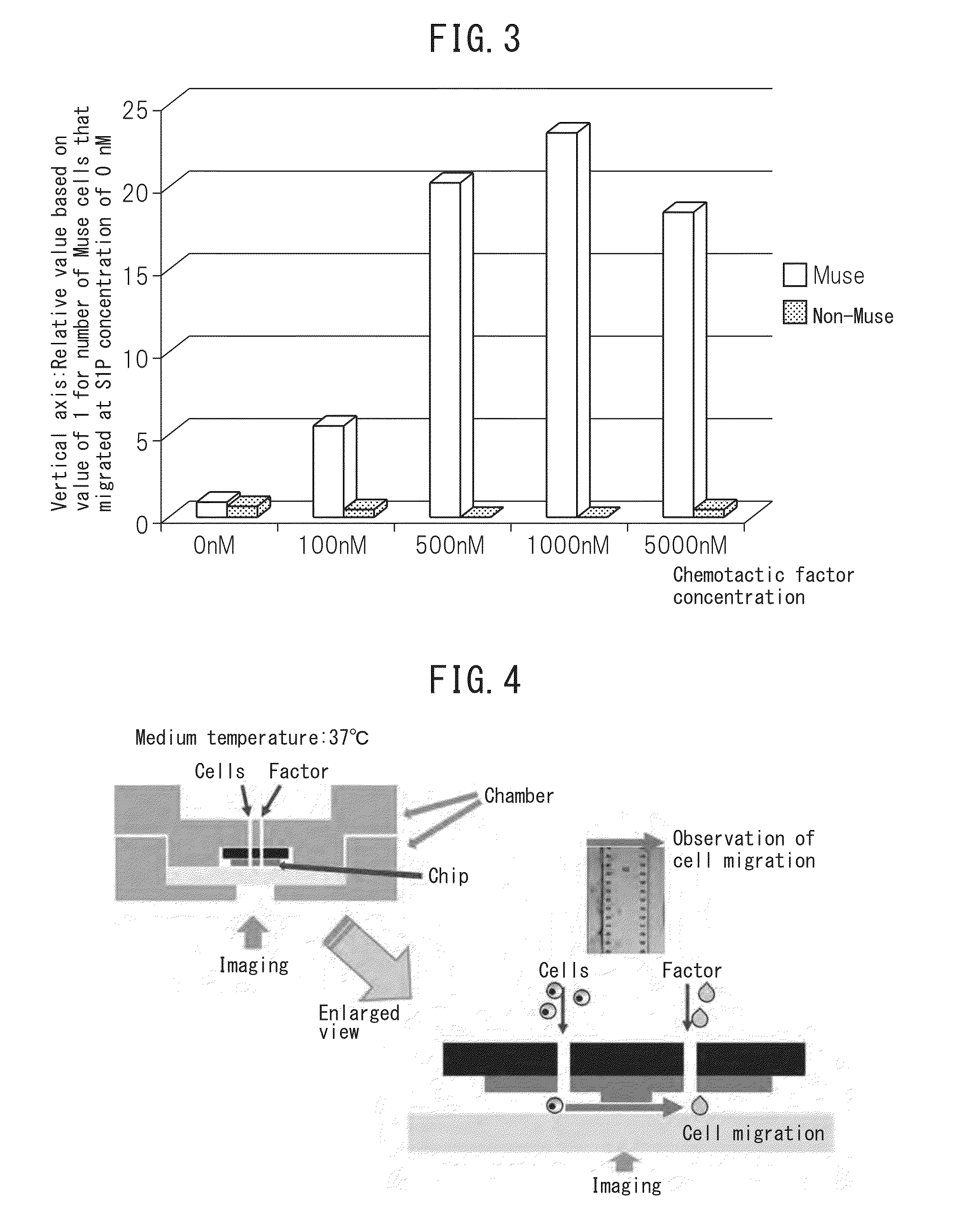
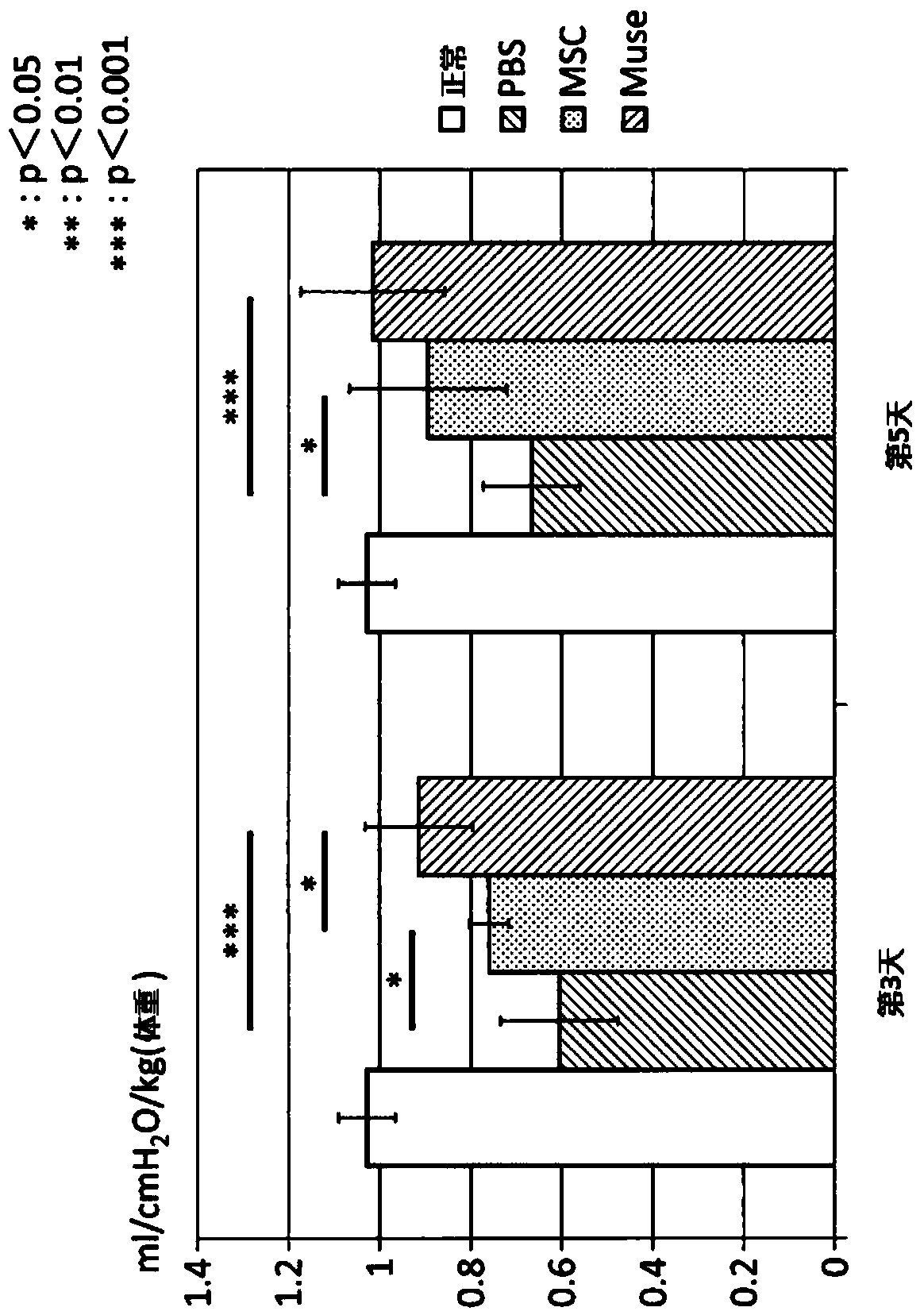
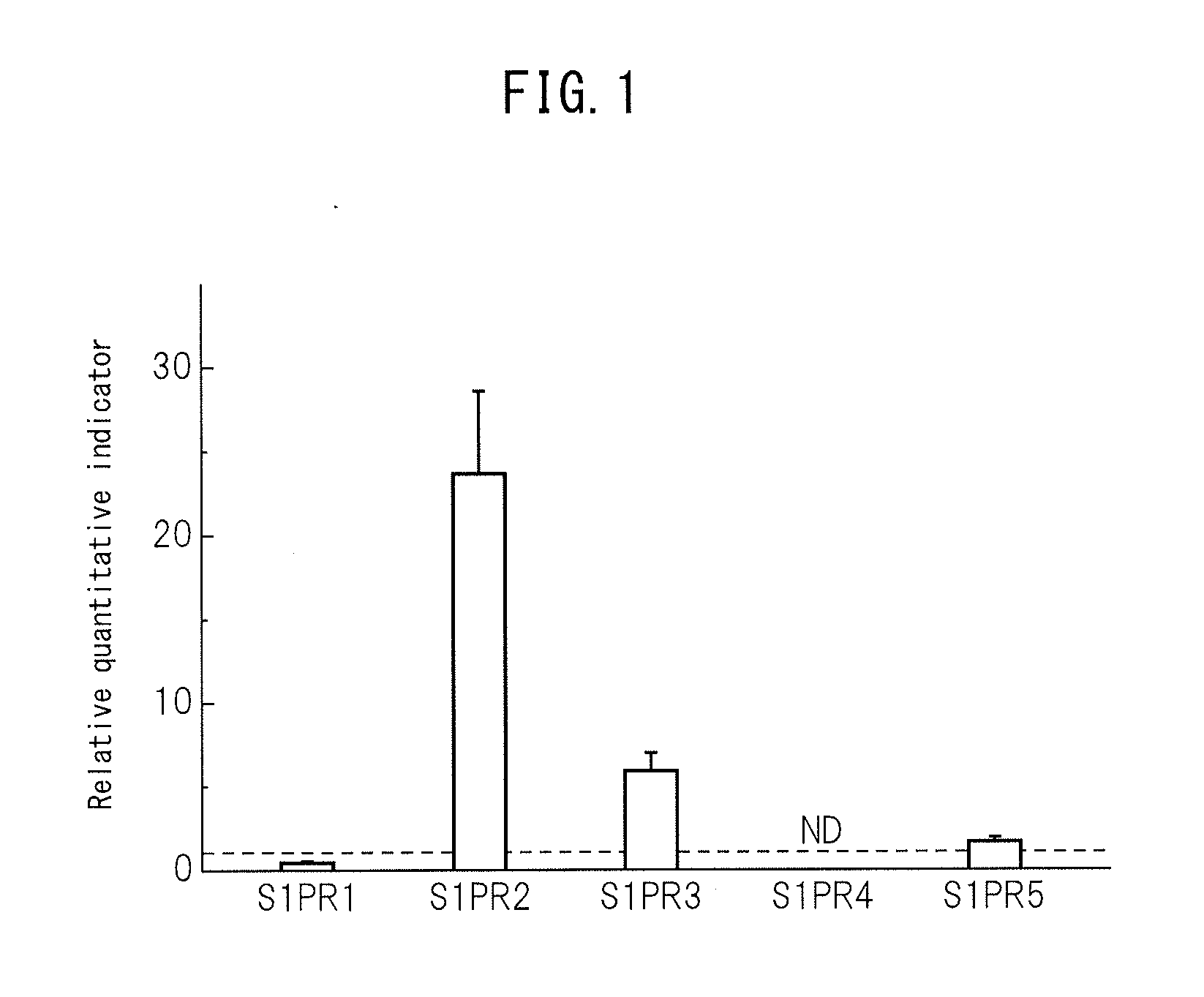
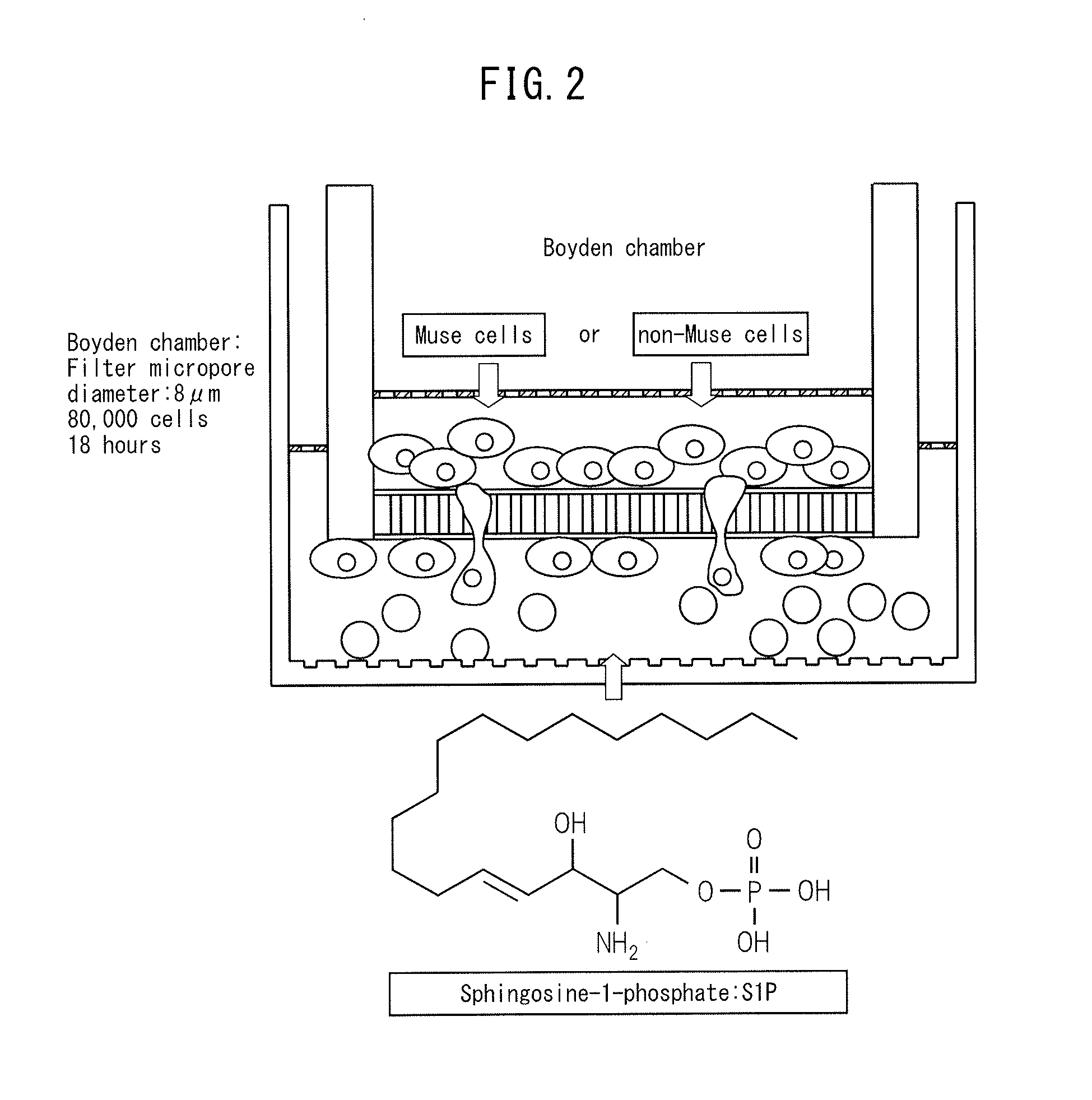
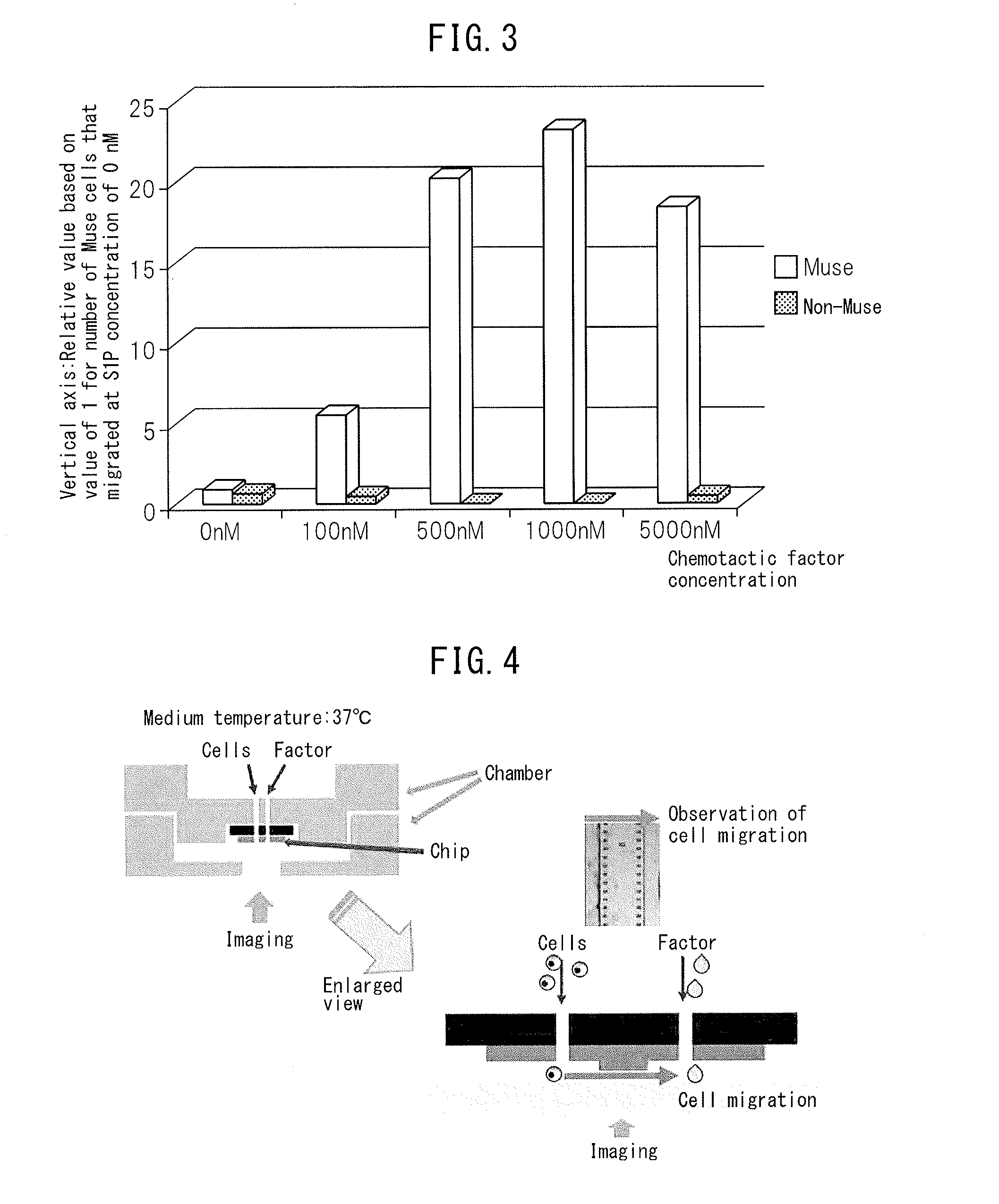
The purpose of the present invention is to identify a migratory factor that guides pluripotent stem cells (Muse cells) useful in new medical applications to damage, and to provide a pharmaceutical composition that includes the migratory factor for promoting tissue regeneration in regenerative medicine that makes use of Muse cells. In the present invention, a receptor that is specifically expressed in Muse cells rather than non-Muse cells was identified, and it was confirmed that a ligand for this receptor can function as a migratory factor. In the present invention, sphingosine-1-phosphate (S1P) was identified as a migratory factor, and thus, the present invention pertains to a pharmaceutical composition for guiding pluripotent stem cells to damage, the composition including S1P as an active ingredient.
Owner:CLIO +2
Pharmaceutical composition including migratory factor for guiding pluripotent stem cells to injury
ActiveUS9446033B2High activityEasily damagedBiocideAntipyreticBULK ACTIVE INGREDIENTActive ingredient
The purpose of the present invention is to identify a migratory factor that guides pluripotent stem cells (Muse cells) useful in new medical applications to damage, and to provide a pharmaceutical composition that includes the migratory factor for promoting tissue regeneration in regenerative medicine that makes use of Muse cells. In the present invention, a receptor that is specifically expressed in Muse cells rather than non-Muse cells was identified, and it was confirmed that a ligand for this receptor can function as a migratory factor. In the present invention, sphingosine-1-phosphate (S1P) was identified as a migratory factor, and thus, the present invention pertains to a pharmaceutical composition for guiding pluripotent stem cells to damage, the composition including S1P as an active ingredient.
Owner:NAT UNIV CORP TOKAI NAT HIGHER EDUCATION & RES SYST +2
Pluripotent stem cell for treatment of cerebral infarction
ActiveUS20170128494A1Reduce infarct sizeImprove brain functionNervous disorderUnknown materialsNeurulationParenchyma
An object of the present invention is to provide a novel medical application to regenerative medicine that uses pluripotent stem cells (Muse cells). The present invention provides a cell preparation for treating cerebral infarction and sequelae associated therewith that contains SSEA-3-positive pluripotent stem cells isolated from mesenchymal tissue in the body or cultured mesenchymal cells. The cell preparation of the present invention is based on a brain tissue regeneration mechanism by which Muse cells differentiate into nerve cells and the like in damaged brain tissue by administering Muse cells into cerebral parenchyma.
Owner:LIFE SCI INST INC +1
Pluripotent stem cell for treating diabetic skin ulcer
InactiveUS20170258844A1Inhibit progressMetabolism disorderMammal material medical ingredientsDiseaseSkin ulcerations
The purpose of the present invention is to provide a novel medicinal use in regeneration medicine, said medicinal use comprising using pluripotent stem cells (Muse cells). Provided is a cell preparation for treating skin ulcer, said cell preparation comprising SSEA-3 positive pluripotent stem cells isolated from a mesenchymal tissue of a living organism or cultured mesenchymal cells. The cell preparation according to the present invention is based on such mechanism of skin tissue regeneration that, when the Muse cells are administered to a skin ulcer site of a subject suffering from the aforesaid disease, the Muse cells differentiate into skin-constituting cells.
Owner:THE UNIV OF TOKYO +1
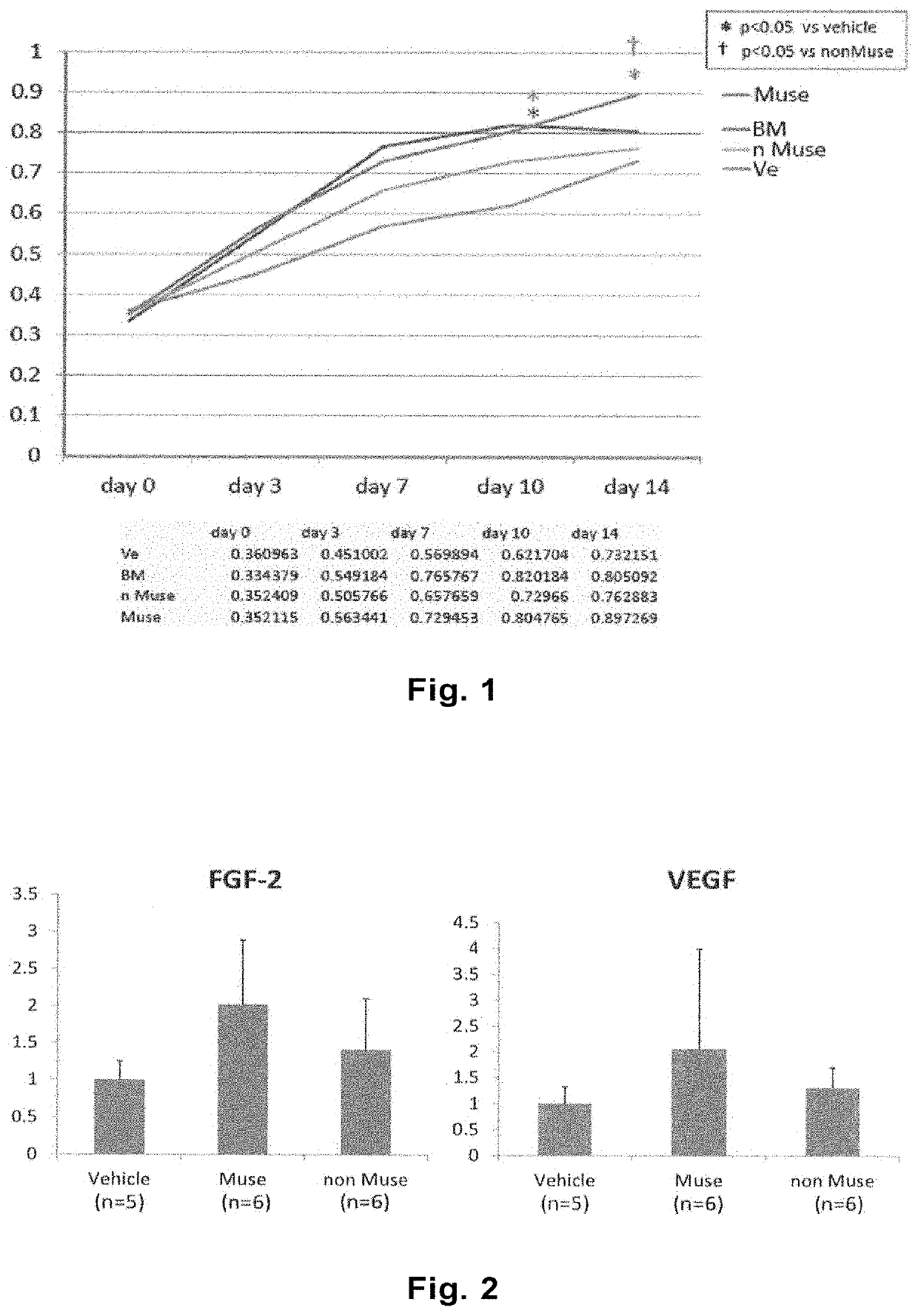
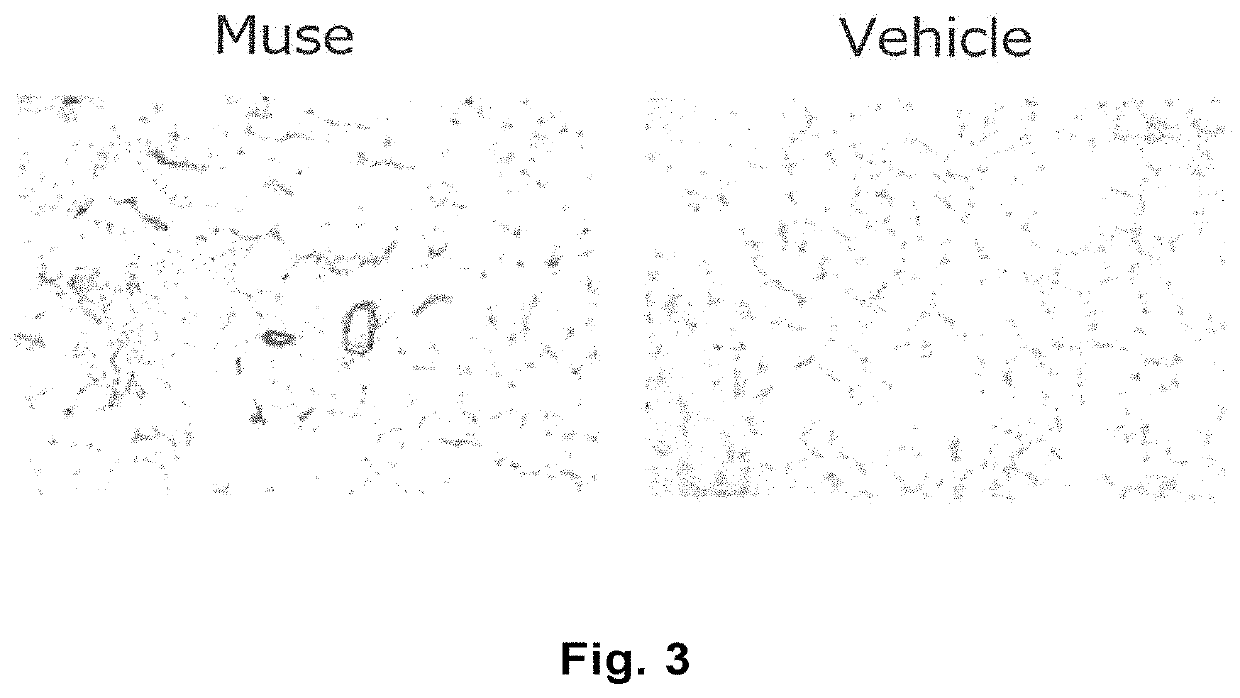
Alleviation and treatment of ischemia reperfusion-induced lung injury using pluripotent stem cells
The purpose of the present invention is to provide a novel medical use of pluripotent stem cells (Muse cells) in regenerative medicine. The present invention provides a cell preparation and a pharmaceutical composition which contain SSEA-3 positive pluripotent stem cells isolated from mesenchymal tissue of a living organism or cultured mesenchymal cells, and which are intended for alleviation andtreatment of ischemia reperfusion-induced lung injury. The cell preparation according to the present invention is based on a mechanism in which Muse cells are administered to a subject having the abovementioned disease to engraft the cells into lung tissues, and the disease is alleviated and treated.
Owner:矢吹皓 +2
Pharmaceutical composition including migratory factor for guiding pluripotent stem cells to damage
ActiveUS20160354393A1High activityAntipyreticAnalgesicsPluripotential stem cellBULK ACTIVE INGREDIENT
Owner:LIFE SCI INST INC +2
Amelioration and treatment of perinatal brain damage with pluripotent stem cells
PendingCN109152801AShrinking Perinatal Brain InjuryNervous disorderDigestive systemInjury brainLearning disability
The present invention addresses the problem of providing a novel use of pluripotent stem cells (Muse cells) for medical purposes in regenerative medicine. The present invention provides a cell preparation and a pharmaceutical composition both for ameliorating and treating perinatal brain damage including learning disability and motor disability, each of the cell preparation and the pharmaceuticalcomposition containing SSEA-3-positive pluripotent stem cells separated from a mesenchymal tissue collected from a living body or cultured mesenchymal cells. The cell preparation according to the present invention relies on a mechanism that Muse cells are administered to a subject having the above-mentioned damage to cause the engraftment of the Muse cells in a damaged brain tissue, thereby ameliorating and treating the damage.
Owner:NAGOYA UNIVERSITY +1
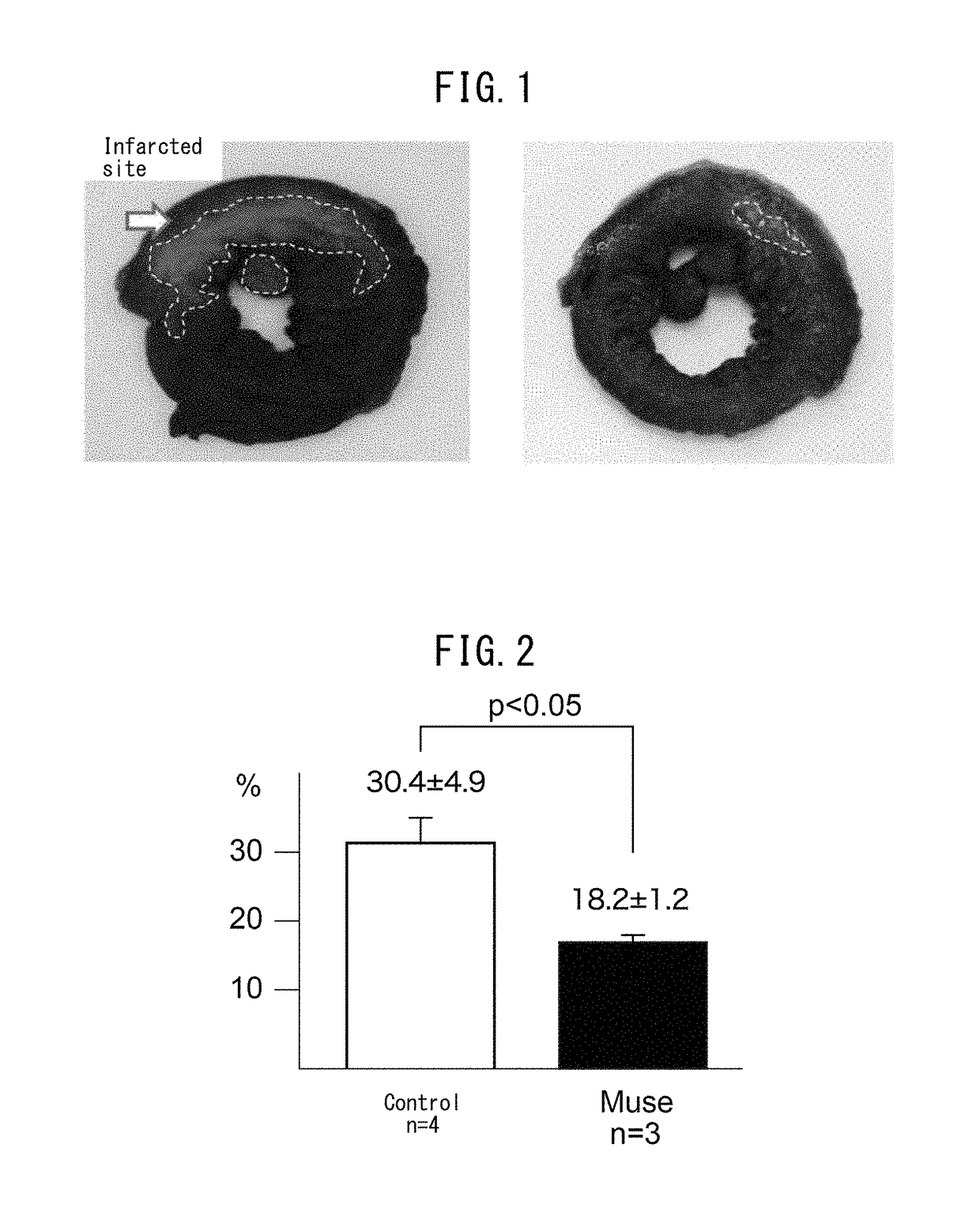
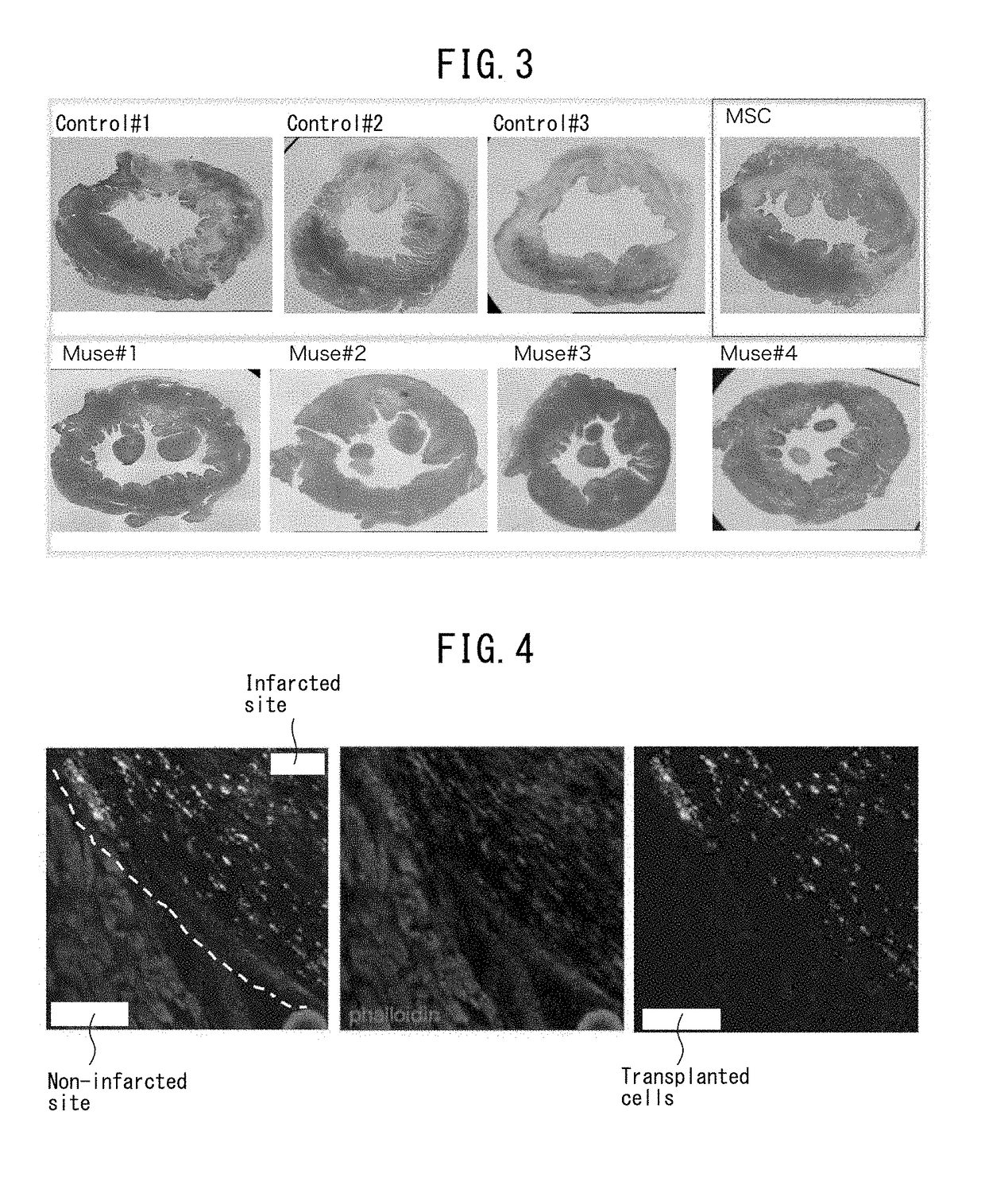
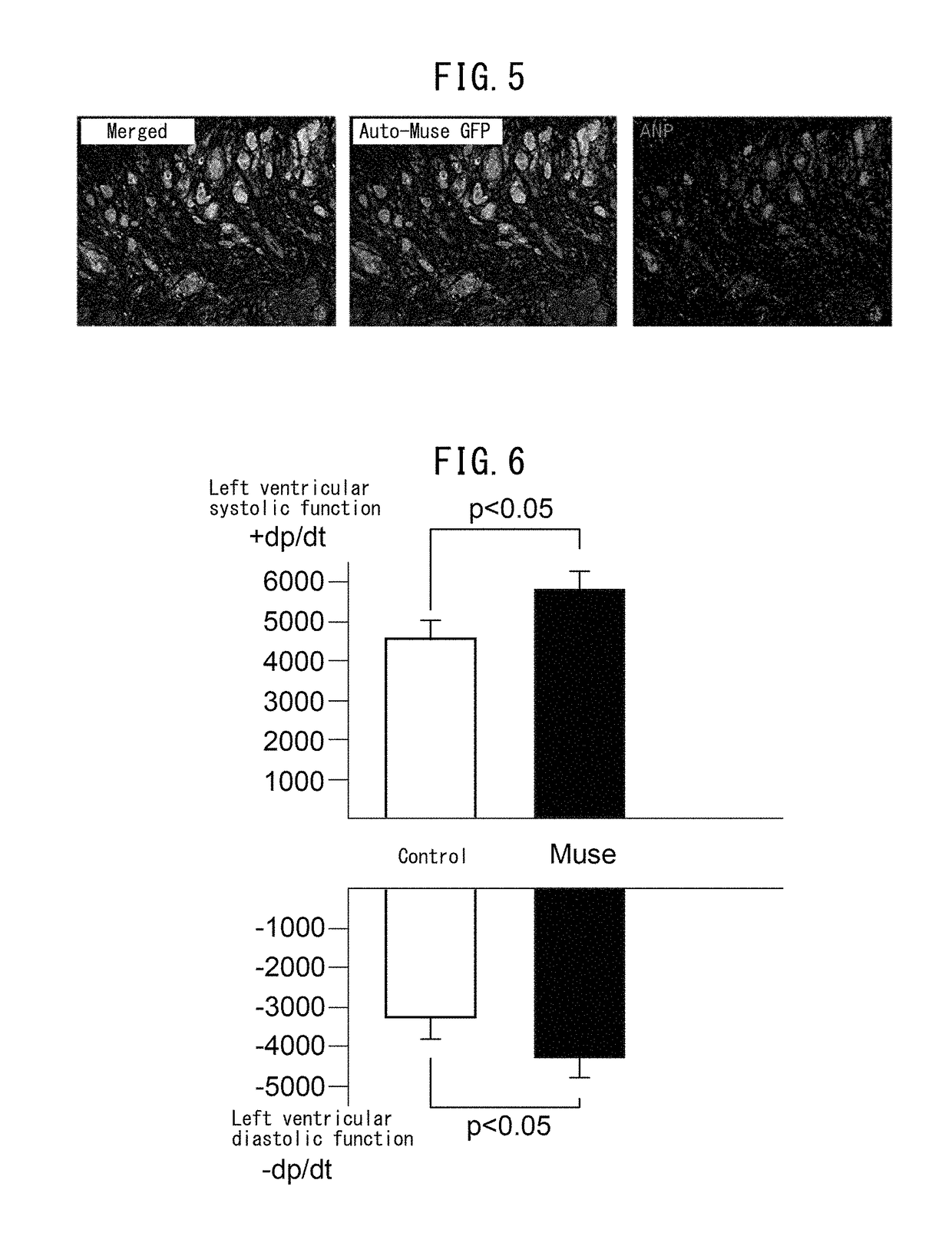
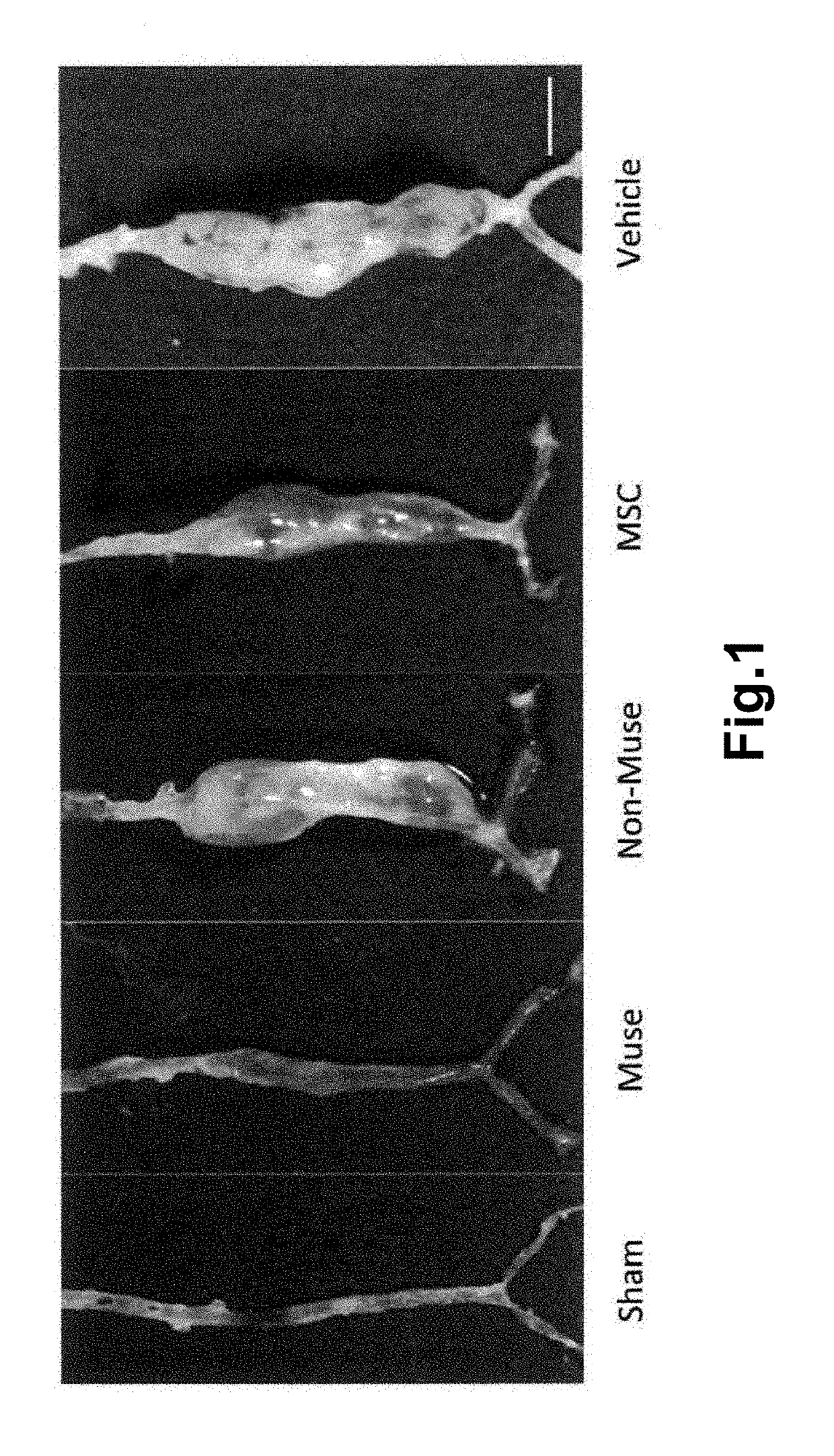
Pluripotent stem cell that induces repair and regeneration after myocardial infarction
ActiveUS9844570B2Small sizeSerious myocardial infarctionGenetic material ingredientsPharmaceutical delivery mechanismDiseaseCardiac muscle
An object of the present invention is to provide a novel medical application for use in regenerative medicine that uses pluripotent stem cells (Muse cells). The present invention provides a cell preparation for treating myocardial infarction, and particularly serious massive myocardial infarction and heart failure associated therewith, that contains pluripotent stem cells positive for SSEA-3 isolated from biological mesenchymal tissue or cultured mesenchymal cells. The cell preparation of the present invention is based on a cardiac tissue regeneration mechanism by which Muse cells are made to selectively accumulate in damaged myocardial tissue and differentiate into cardiac muscle in that tissue as a result of intravenous administration of Muse cells to a subject presenting with the aforementioned disorders.
Owner:CLIO +2
Prophylactic or therapeutic agent for organ fibrosis
PendingCN109689073AFunction increaseImprove securityNervous disorderDigestive systemLiver fibrosisOrgan of Zuckerkandl
The present invention addresses the problem of providing a safe and easy-to-prepare cell preparation for prevention and / or treatment of organ fibrosis such as liver fibrosis. Provided is a cell preparation for prevention and / or treatment of organ fibrosis such as liver fibrosis, the cell preparation including a SSEA-3-positive pluripotent stem cell (Muse cell) derived from a vital mesenchymal tissue or a cultured mesenchymal cell.
Owner:TOHOKU UNIV
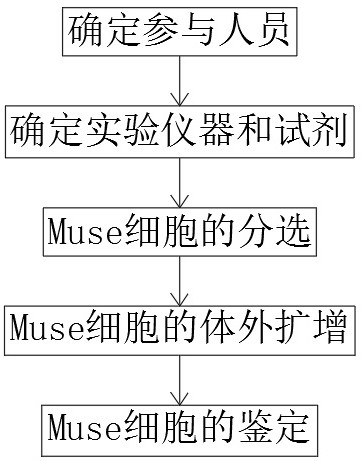
Method for culturing human umbilical cord-derived Muse cells
InactiveCN112852727AHigh purityReasonable designCell dissociation methodsSkeletal/connective tissue cellsCD29Proliferative capacity
The invention discloses a culture method of human umbilical cord-derived Muse cells. The culture method comprises the following steps: determining participants, determining experimental instruments and reagents, determining an experimental method, separating the Muse cells, and performing in-vitro amplification culture on the Muse cells. The method has the beneficial effects that by applying the culture system, the MSCs obtained by separation are in a typical fibroblast form and have relatively strong multiplication capacity. According to the Muse cell culture method disclosed by the invention, the Muse cells are in positive expression, CD29, CD44, CD90 and CD105 are all in positive expression, and CD34 and CD45 are in negative expression, so that the Muse cells with relatively high purity can be obtained by the culture method, and a certain reference is provided for clinical application of the Muse cells in mesenchymal stem cell treatment, tissue regeneration, immunoregulation and the like in future.
Owner:HENAN YINFENG BIOENG CO LTD +1
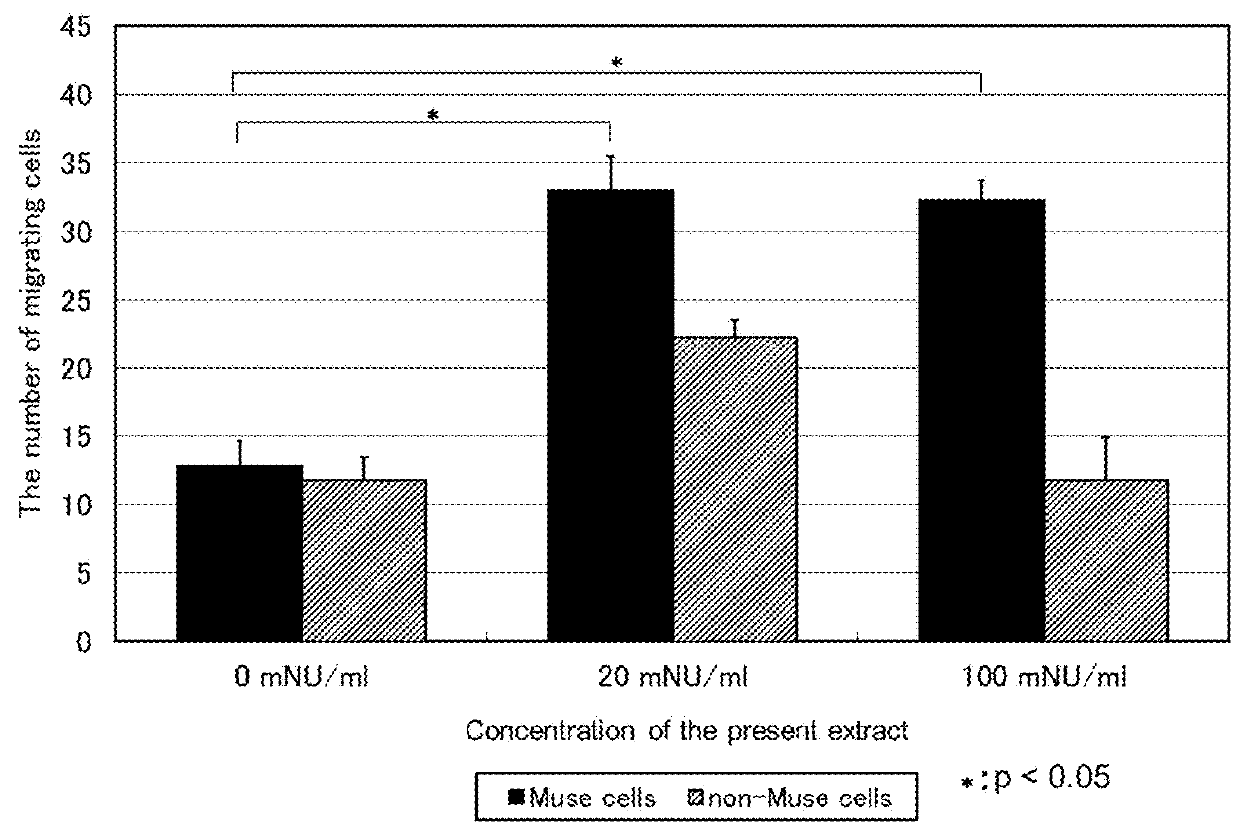
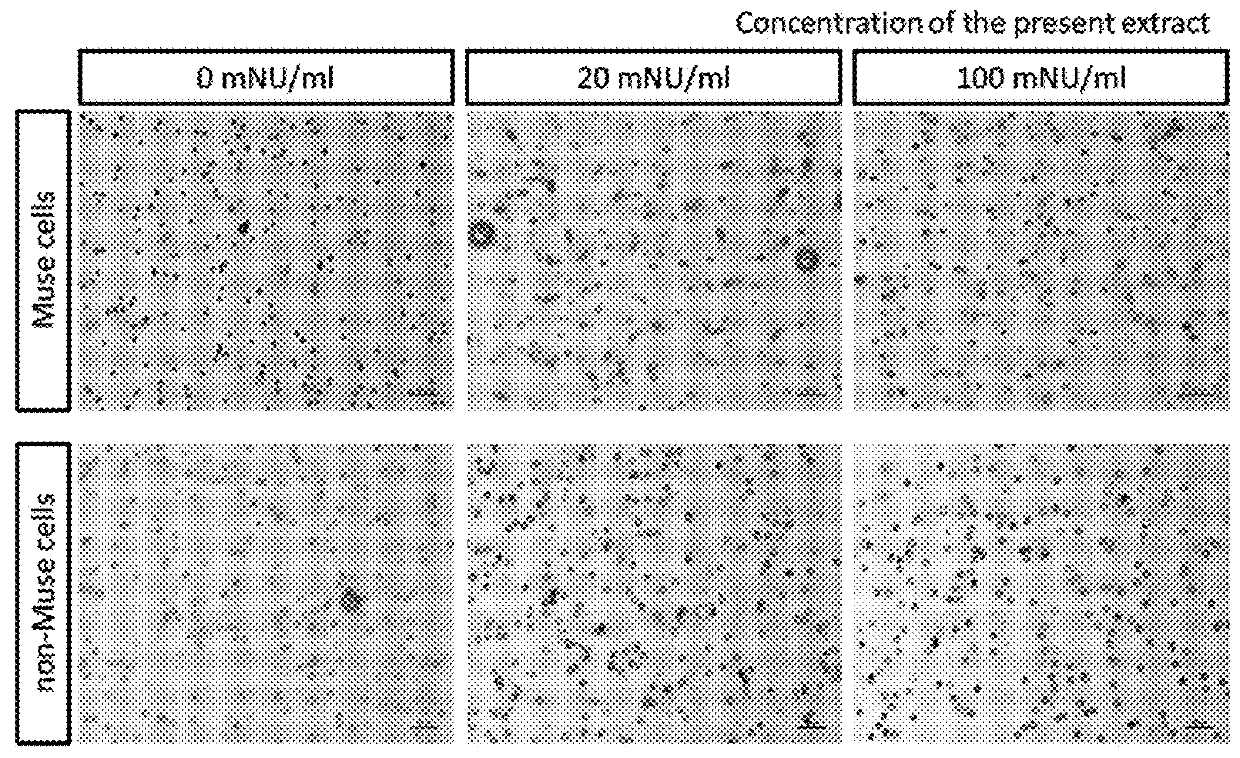
Agent for promoting migration of pluripotent stem cells
InactiveUS20200254026A1Promote migrationEnhance pharmacological effectsCulture processPharmaceutical delivery mechanismMedicinePox viruses
An agent for promoting migration of pluripotent stem cells containing an extract from inflamed tissues inoculated with vaccinia virus. An extract from inflamed tissues inoculated with vaccinia virus promotes migration of Muse cells. Thus, the agent for promoting migration of pluripotent stem cells for various diseases to which migration of pluripotent stem cells may have an advantageous effect.
Owner:NIPPON ZOKI PHARM CO LTD
Treatment agent for epidermolysis bullosa
PendingUS20200206272A1Ameliorating and healing skin symptomImprove securityEpidermal cells/skin cellsSkeletal/connective tissue cellsBiochemistryLiving body
A cell product for treatment of epidermolysis bullosa, comprising a SSEA-3-positive pluripotent stem cell (Muse cell) derived from a mesenchymal tissue in a living body or a cultured mesenchymal cell. Preferably, the epidermolysis bullosa is epidermolysis bullosa simplex, junctional epidermolysis bullosa, or dystrophic epidermolysis bullosa.
Owner:HOKKAIDO UNIVERSITY
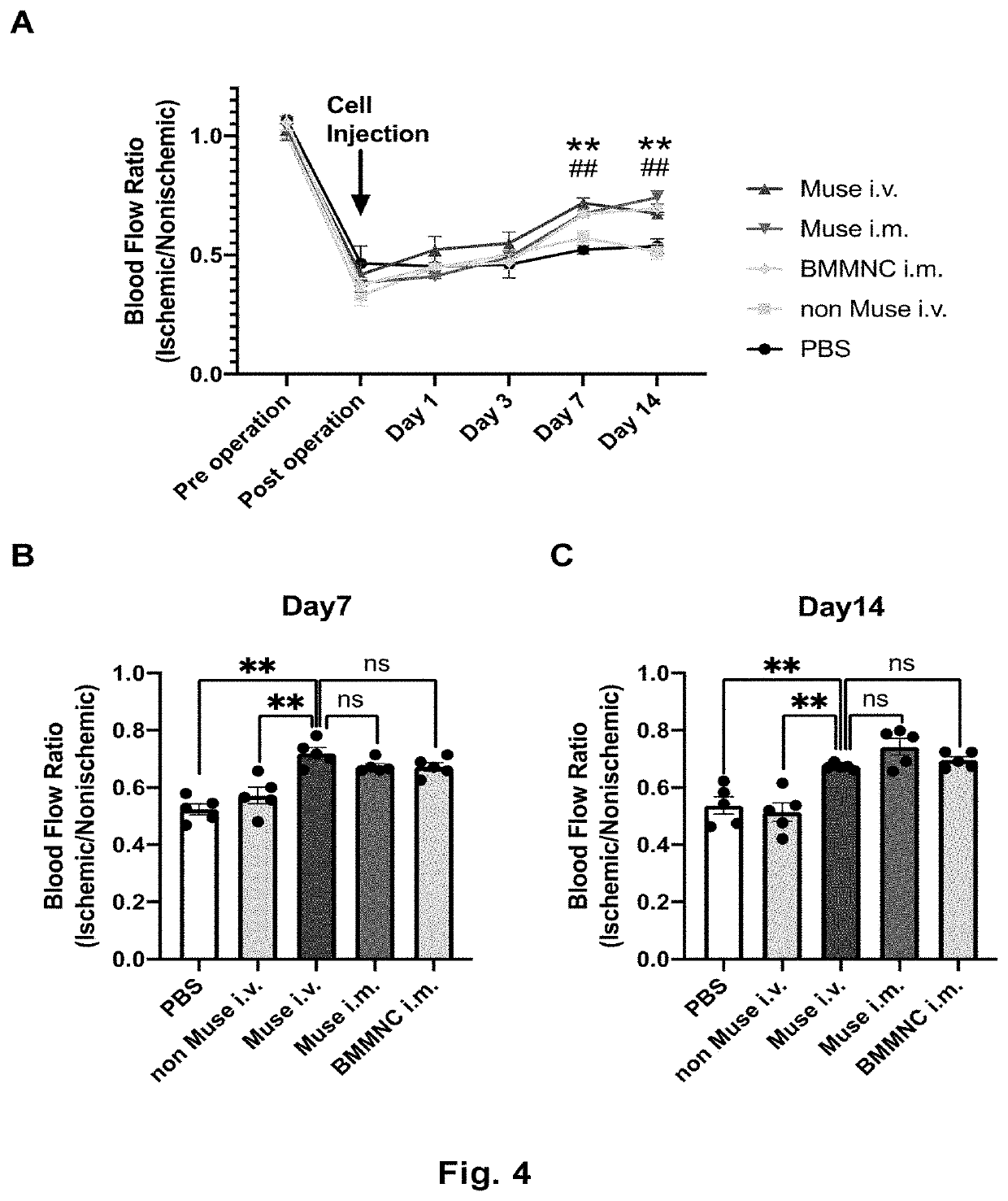
Therapeutic agent of peripheral blood flow disorder
PendingCN113195054AImprovement or restoration of blood flow disturbanceImprove securityUnknown materialsCardiovascular disorderPluripotential stem cellPeripheral blood flow
Provided is a cell preparation for treating a peripheral blood flow disorder, the cell preparation including pluripotent stem cells (Muse cells) which are SSEA-3 positive and derived from mesenchymal tissues of a living body or cultured mesenchymal cells. The peripheral blood flow disorder is preferably a peripheral arterial disease, and more preferably, chronic arterial obstruction of the limbs.
Owner:KYOTO PREFECTURAL PUBLIC UNIV CORP
Pluripotent stem cell that induces repair and regeneration after myocardial infarction
ActiveUS20180050067A1Small sizeSerious myocardial infarctionPharmaceutical delivery mechanismUnknown materialsDiseaseVein
An object of the present invention is to provide a novel medical application for use in regenerative medicine that uses pluripotent stem cells (Muse cells). The present invention provides a cell preparation for treating myocardial infarction, and particularly serious massive myocardial infarction and heart failure associated therewith, that contains pluripotent stem cells positive for SSEA-3 isolated from biological mesenchymal tissue or cultured mesenchymal cells. The cell preparation of the present invention is based on a cardiac tissue regeneration mechanism by which Muse cells are made to selectively accumulate in damaged myocardial tissue and differentiate into cardiac muscle in that tissue as a result of intravenous administration of Muse cells to a subject presenting with the aforementioned disorders.
Owner:TOHOKU UNIV +2
Prophylactic or therapeutic agent for vascular disorder
ActiveUS20190175662A1Improvement and recovery of functionEffect is exertedUnknown materialsSkeletal/connective tissue cellsVascular diseaseLiving body
A cell product for prevention and / or treatment of vascular disorders such as aortic aneurysm, comprising a SSEA-3-positive pluripotent stem cell derived from a mesenchymal tissue in a living body or a cultured mesenchymal cell (Muse cell).
Owner:TOHOKU UNIV +1
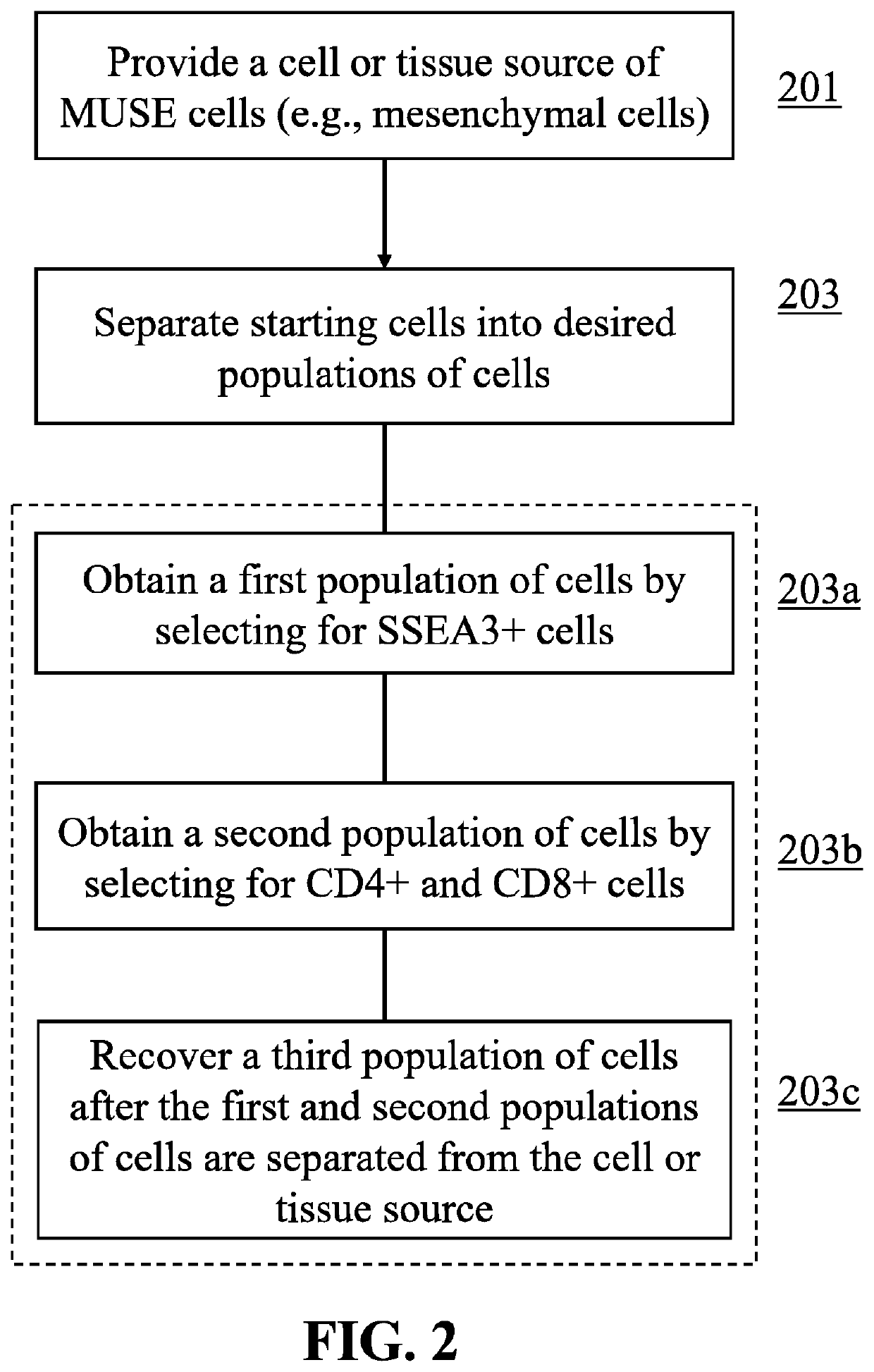
Methods for enriching populations of cells
PendingUS20220169979A1Nervous disorderMammal material medical ingredientsHereditary disordersCell biology
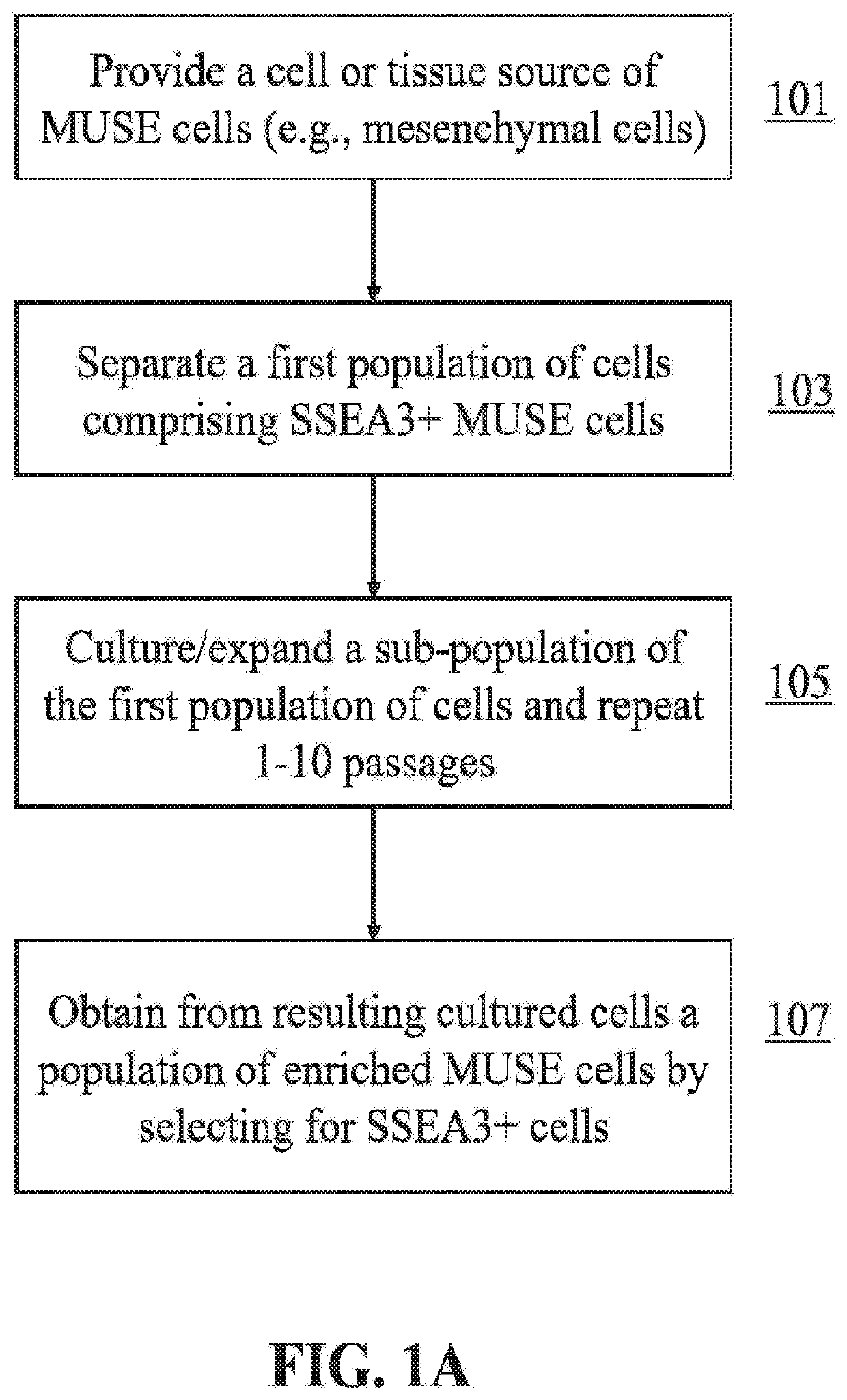
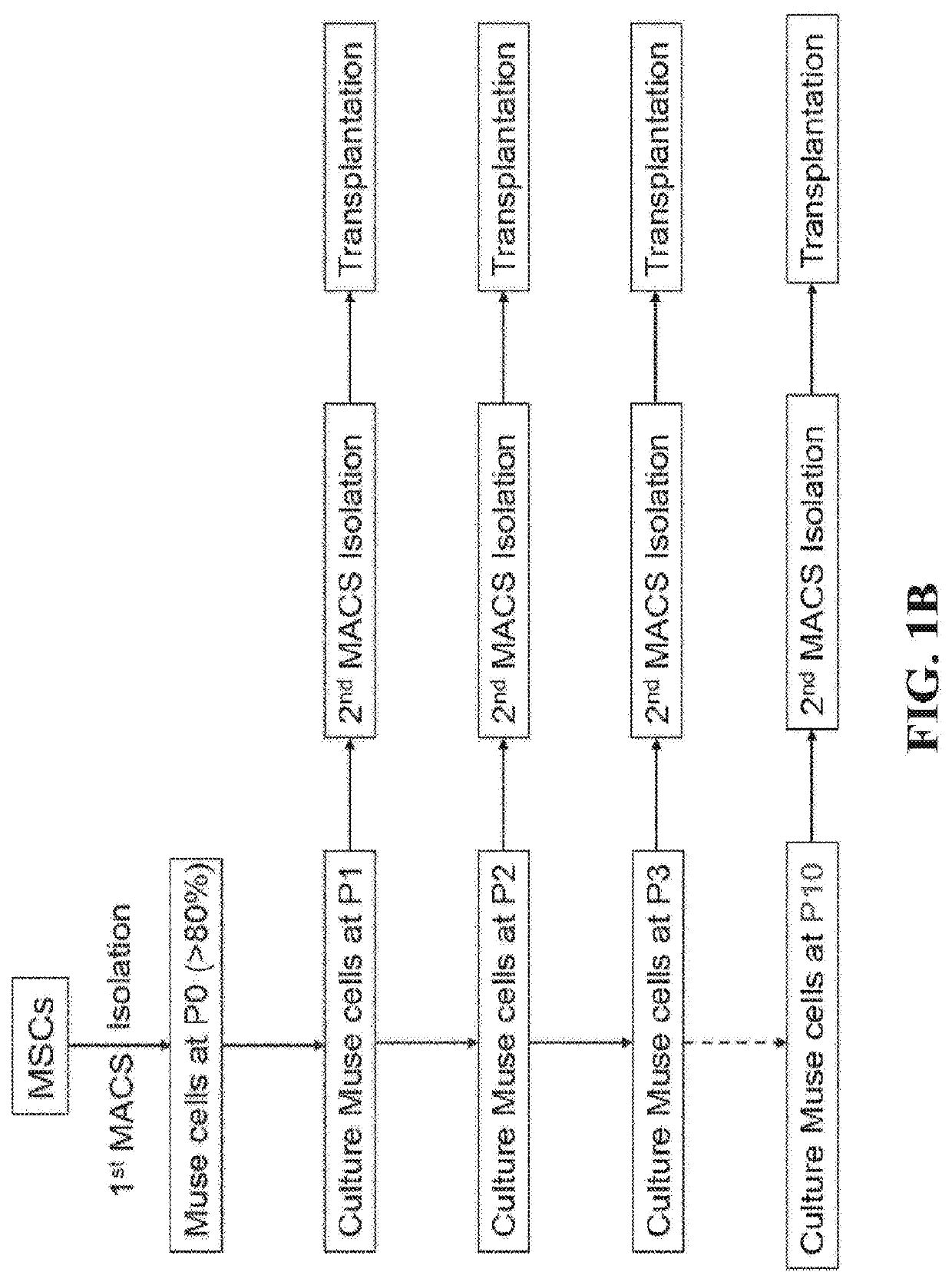
This disclosure describes efficient methods for separating desired populations of cells, including Multilineage-Differentiating Stress-Enduring (MUSE) cells. Also described are the methods for isolating and enriching MUSE cells through a sorting, expanding, and re-sorting procedure. The enriched cells or cell populations can be used for treating cancer, repairing various tissues, and treating various degenerative or inherited diseases.
Owner:RUTGERS THE STATE UNIV
Agent for promoting migration of pluripotent stem cells
InactiveUS20180161378A1Promote migrationEnhance pharmacological effectsCulture processPharmaceutical delivery mechanismDiseaseVaccinia
An agent for promoting migration of pluripotent stem cells containing an extract from inflamed tissues inoculated with vaccinia virus. An extract from inflamed tissues inoculated with vaccinia virus promotes migration of Muse cells. Thus, the agent for promoting migration of pluripotent stem cells for various diseases to which migration of pluripotent stem cells may have an advantageous effect.
Owner:NIPPON ZOKI PHARM CO LTD
Methods for enriching populations of cells
PendingCN114026221ANervous disorderMammal material medical ingredientsHereditary disordersCell biology
This disclosure describes efficient methods for separating desired populations of cells, including Multilineage-Differentiating Stress-Enduring (MUSE) cells. Also described are the methods for isolating and enriching MUSE cells through a sorting, expanding, and re-sorting procedure. The enriched cells or cell populations can be used for treating cancer, repairing various tissues, and treating various degenerative or inherited diseases.
Owner:RUTGERS THE STATE UNIV
Multilineage-differentiating stress enduring (MUSE) cells for treatment of chronic kidney disease
ActiveUS10293003B2Promote recoveryImprove kidney functionUnknown materialsUrinary disorderDiseaseVein
An object of the present invention is to provide a novel medical application to regenerative medicine that uses pluripotent stem cells (Muse cells). The present invention provides a cell preparation for treating chronic kidney disease that contains SSEA-3-positive pluripotent stem cells isolated from mesenchymal tissue in the body or cultured mesenchymal cells. The cell preparation of the present invention is based on a renal tissue regeneration mechanism by which Muse cells are made to selectively accumulate at a site of kidney disease and differentiate into cells that compose the kidney by administering Muse cells intravenously to a subject having the aforementioned disease.
Owner:LIFE SCI INST INC +1
Screening and identification method of human umbilical cord-derived Muse cells
InactiveCN113106058AReasonable designCell dissociation methodsMicrobiological testing/measurementOsteoblastAdipogenesis
The invention discloses a screening and identification method of human umbilical cord-derived Muse cells. The method comprises the following steps: determining participants, determining experimental instruments and reagents, sorting the Muse cells, performing in-vitro amplification on the Muse cells, and identifying the Muse cells. The method disclosed by the invention has the beneficial effects that positive cells of the primarily cultured dermal fibroblasts SSEA-3 are sorted by a flow cytometer, the Muse cell cluster is cultured under the conditions of an MC culture medium and a poly-HEMA coated plate, and the self-renewal capacity of suspension-adherence-suspension and proliferation amplification of the Muse cell cluster is observed. The cells can be differentiated into adipocytes and osteoblasts through bone and adipogenesis induction of the third generation Muse cells. It is proved that a human umbilical cord source Muse cell culture scheme is successfully established in the project.
Owner:HENAN YINFENG BIOENG CO LTD +1
Pluripotent stem cell for treatment of chronic kidney disease
ActiveUS20170128498A1Promote recoveryImprove kidney functionUnknown materialsUrinary disorderDiseaseRenal tissue
Owner:LIFE SCI INST INC +1
Therapeutic agent of peripheral blood flow disorder
PendingUS20220000936A1Ameliorating and treating blood flow disorderImprove securityUnknown materialsCardiovascular disorderPeripheral blood flowArterial obstruction
A cell product may be used for treating a peripheral blood flow disorder, and the cell product may include an SSEA-3-positive pluripotent stem cells (Muse cells) derived from a mesenchymal tissue in a living body or a cultured mesenchymal cell. The peripheral blood flow disorder is preferably a peripheral arterial disease, more preferably chronic arterial obstruction in a limb.
Owner:KYOTO PREFECTURAL PUBLIC UNIV CORP +1
Treatment agent for epidermolysis bullosa
PendingCN110831607AImprove or restore symptomsRebuilds and repairs damaged skinEpidermal cells/skin cellsArtificial cell constructsMedicineBiochemistry
Provided is a cell preparation for treating epidermolysis bullosa, containing SSEA-3 positive pluripotent stem cells (muse cells) derived from a mesenchymal tissue of a live body or cultured mesenchymal cells. The epidermolysis bullosa is preferably epidermolysis bullosa simplex, junctional epidermolysis bullosa, or dystrophic epidermolysis bullosa.
Owner:HOKKAIDO UNIVERSITY +1
Therapeutic agent for spinal cord injury
PendingUS20210228638A1Improving and restoring motor functionImprove securityNervous disorderMuscular disorderSpinal cord lesionAnatomy
A cell product for treatment of spinal cord injury, comprising a SSEA-3-positive pluripotent stem cell (Muse cell) derived from a mesenchymal tissue in a living body or a cultured mesenchymal cell. Preferably, the spinal cord injury is complete or incomplete spinal cord injury.
Owner:LIFE SCI INST INC
Pluripotent stem cell for treating diabetic skin ulcer
ActiveUS20190192574A1Inhibit progressMetabolism disorderMammal material medical ingredientsDiseaseBiological body
The purpose of the present invention is to provide a novel medicinal use in regeneration medicine, said medicinal use comprising using pluripotent stem cells (Muse cells). Provided is a cell preparation for treating skin ulcer, said cell preparation comprising SSEA-3 positive pluripotent stem cells isolated from a mesenchymal tissue of a living organism or cultured mesenchymal cells. The cell preparation according to the present invention is based on such mechanism of skin tissue regeneration that, when the Muse cells are administered to a skin ulcer site of a subject suffering from the aforesaid disease, the Muse cells differentiate into skin-constituting cells.
Owner:THE UNIV OF TOKYO +1
Method of treating aortic aneurysm using muse cells
ActiveUS11419899B2Promote functional recoveryEffect is exertedBiocideSkeletal/connective tissue cellsAnatomyBlood vessel
A cell product for prevention and / or treatment of vascular disorders such as aortic aneurysm, comprising a SSEA-3-positive pluripotent stem cell derived from a mesenchymal tissue in a living body or a cultured mesenchymal cell (Muse cell).
Owner:TOHOKU UNIV
Amelioration and treatment of brain disorder resulting from fetal growth retardation using pluripotent stem cells
PendingCN110869034AReduce brain damageNervous disorderNervous system cellsPluripotential stem cellFetal growth
The purpose of the present invention is to provide a novel medical application of pluripotent stem cells (muse cells) in regeneration medicine. The present invention provides a cell preparation and apharmaceutical composition which are for amelioration and treatment of brain disorders resulting from fetal growth retardation, such as abnormal motor quality or abnormal neurological development, andwhich contain SSEA-3 positive pluripotent stem cells isolated from a mesenchymal tissue from a live body or cultured mesenchymal cells. It is assumed that this cell preparation is based on a mechanism where muse cells that are administered to objects having the disorders are engrafted on an impaired brain tissue, thereby ameliorating or treating the disorders.
Owner:NAGOYA UNIVERSITY +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com