Pluripotent stem cell for treatment of chronic kidney disease
a stem cell and chronic kidney disease technology, applied in the field of chronic kidney disease treatment, can solve the problems of loss of kidney function, increasing patient qol and national medical care expenditure, and increasing the number of patients, so as to improve kidney function and suppress the progression of chronic kidney disorder.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Mouse Chronic Kidney Disease Model
[0056]The “Regulations for Animal Experiments and Related Activities at Tohoku University” were strictly observed for the experimental protocol using mice in the present example, and experimental animals were prepared in accordance with these regulations under the supervision of the Tohoku University Experimental Animal Center. More specifically, a mouse chronic kidney disease model was prepared by administering doxorubicin hydrochloride (Sigma Corp.) into a caudal vein of Balb / c mice and SCID mice (males, age 11 to 13 weeks) at 11.5 μg / g of mouse body weight. These animal models presented with a clinical picture resembling focal segmental glomerular sclerosis (FSGS), which is one of the causative diseases of chronic renal failure in humans.
example 2
Preparation of Human Muse Cells
[0057]Preparation of human Muse cells was carried out in accordance with the method described in International Publication No. WO 2011 / 007900. More specifically, adhesive mesenchymal cells were cultured from human bone marrow fluid and after the cells proliferated, Muse cells or cell populations containing Muse cells were isolated by FACS as SSEA-3-positive cells. In addition, non-Muse cells consisted of a cell group negative for SSEA-3 present among the aforementioned mesenchymal cells, and were used as a control. Subsequently, the cells were adjusted to a prescribed concentration using phosphate-buffered physiological saline or culture liquid, and were used to evaluate the effects of Muse cells on renal function in the chronic kidney disease mouse model as indicated below. Furthermore, in the case of using cells obtained by culturing mesenchymal cells such as bone marrow-derived mesenchymal cells as a population of Muse cells, all SSEA-3-positive cel...
example 3
Evaluation of Renal Function in a Balb / c Mouse
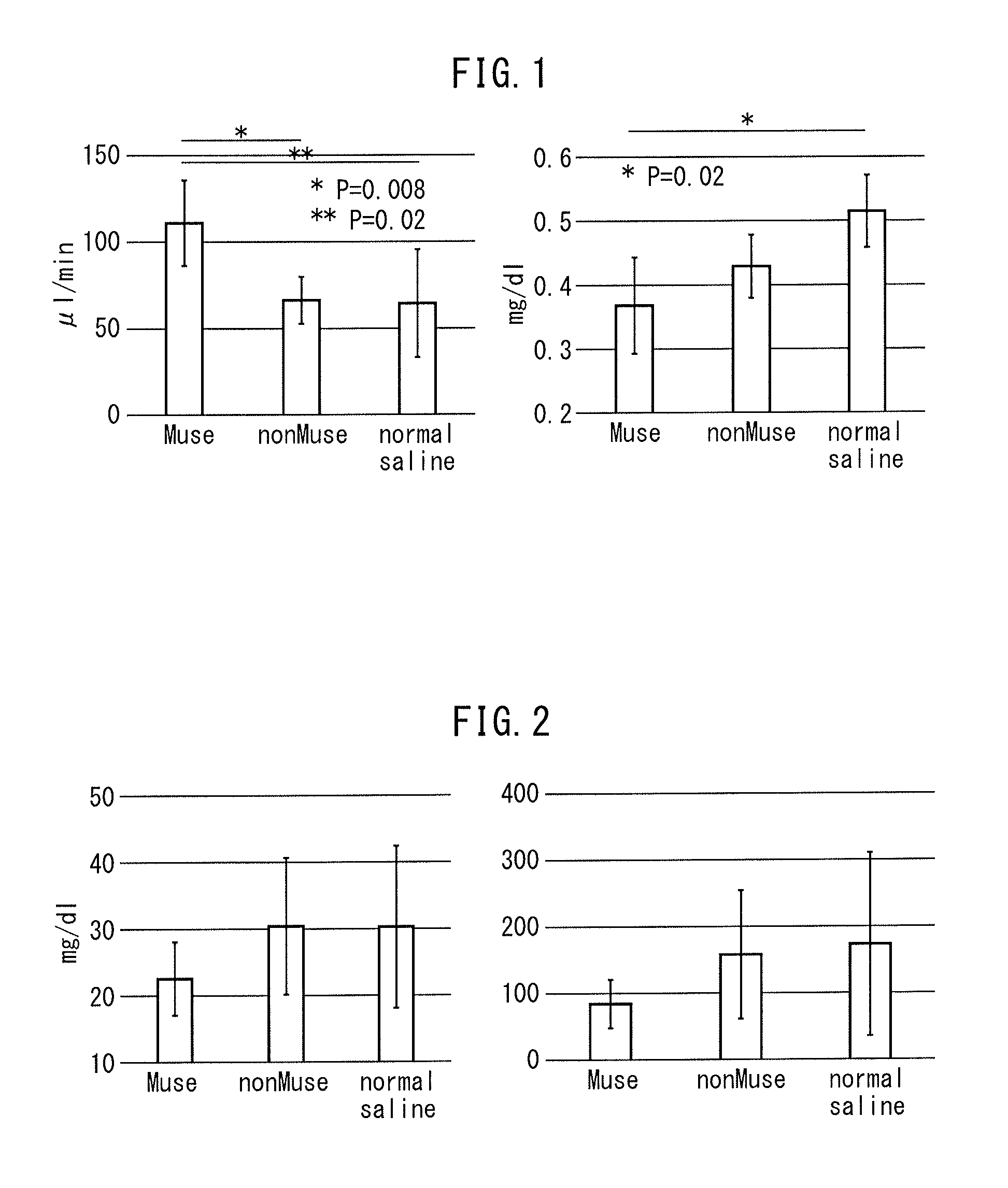
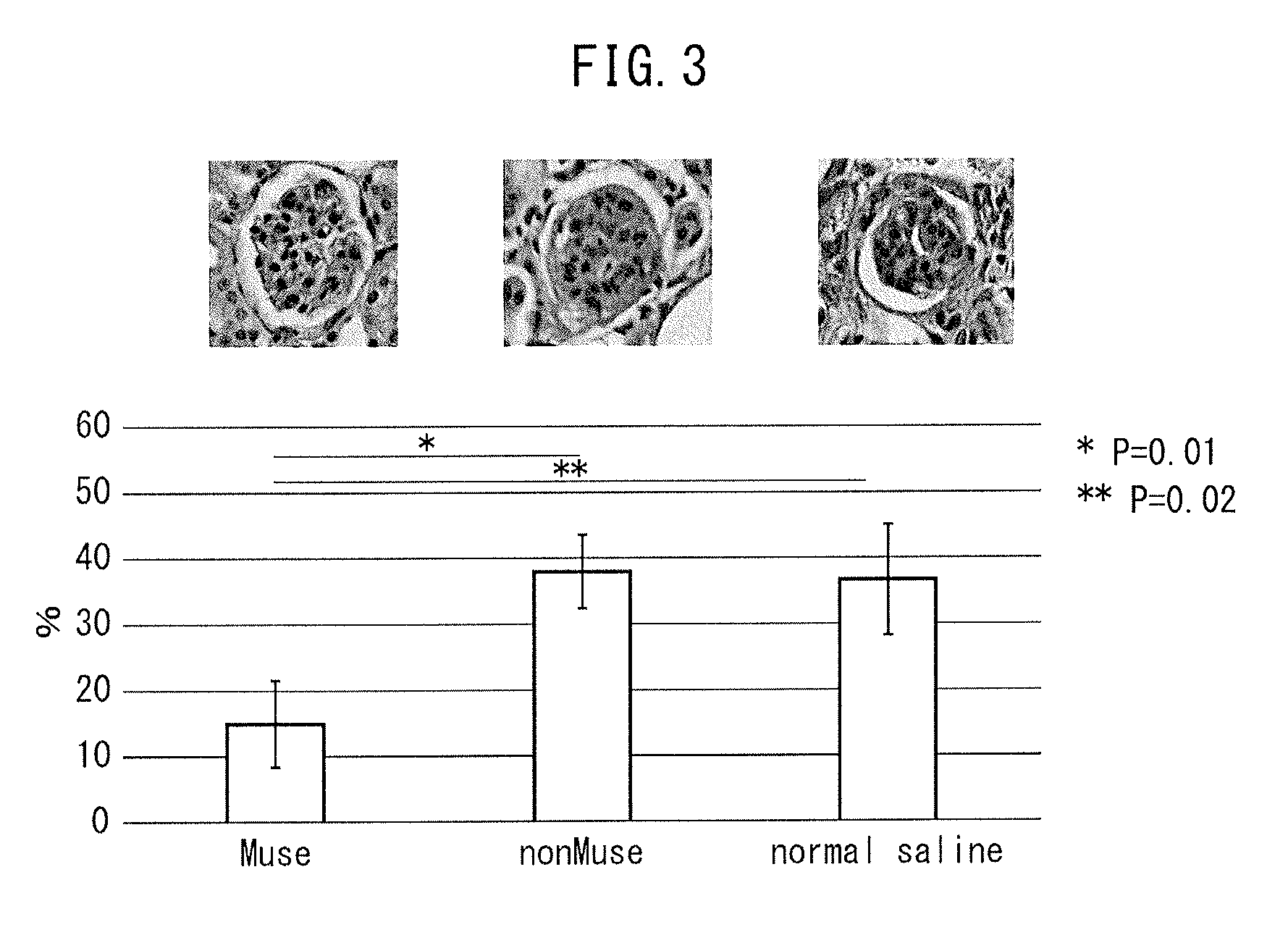
[0058]Chronic Kidney Disease Model by Transplanting Muse Cells The chronic kidney disease mice (Balb / c) prepared in
[0059]Example 1 were divided into three groups, and Muse cells (2×104 cells, 200 μl), human bone marrow-derived non-Muse cells (2×104 cells, 200 μl) or physiological saline (200 μl) were administered into a caudal vein of mice in each group one week after administration of doxorubicin hydrochloride. Subsequently, after allowing the passage of a prescribed amount of time, creatinine clearance, serum creatinine, blood urea nitrogen (BUN) and urine protein were measured in accordance with ordinary methods for each mouse followed by evaluation of the therapeutic effects of Muse cells on the chronic kidney disease mice. Furthermore, although all of the cells used for administration were of human origin, an immunosuppressant was not used throughout the experimental period when the cells were administered to the mice.
(a) Creatinine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com