Pluripotent stem cell for treating diabetic skin ulcer
a stem cell and diabetic technology, applied in the field of regenerative medicine, can solve the problems of increasing the number of patients, increasing the risk of chronic skin ulcers, and affecting the survival of patients with diabetes mellitus, and achieve the effect of inhibiting the progression of skin ulcers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Human Muse Cells
[0068](1) Sampling of Human Tissue and Cell Preparation
[0069]Lipoaspirates were acquired by liposuction surgery from the abdomens and / or thighs of five non-obese women (age: 26.6±8.7 years, BMI: 21.5±2.0) from whom consent was obtained with the approval of the ethics committee of the Graduate School of Medicine and Faculty of Medicine of the University of Tokyo. Stromal vascular fractions (SVF) containing adipose-derived stromal / stem cells (ASC) were isolated from the aspirated adipose tissue as previously described (see Yoshimura, K. et al., J. Cell Physiol., Vol. 208, p. 64-76 (2006)). Simply put, aspirated adipose tissue was washed with PBS and digested in PBS containing 0.075% collagenase for 30 minutes at 37° C. on a shaker. Mature adipocytes and connective tissue were separated from the pellet by centrifugal separation. The cell pellet was then re-suspended and lysed by passing through 100 μm, 70 μm and 40 μm screens. The cell pellet containing a...
example 2
Production of Immunosuppressed Diabetic Mice Model
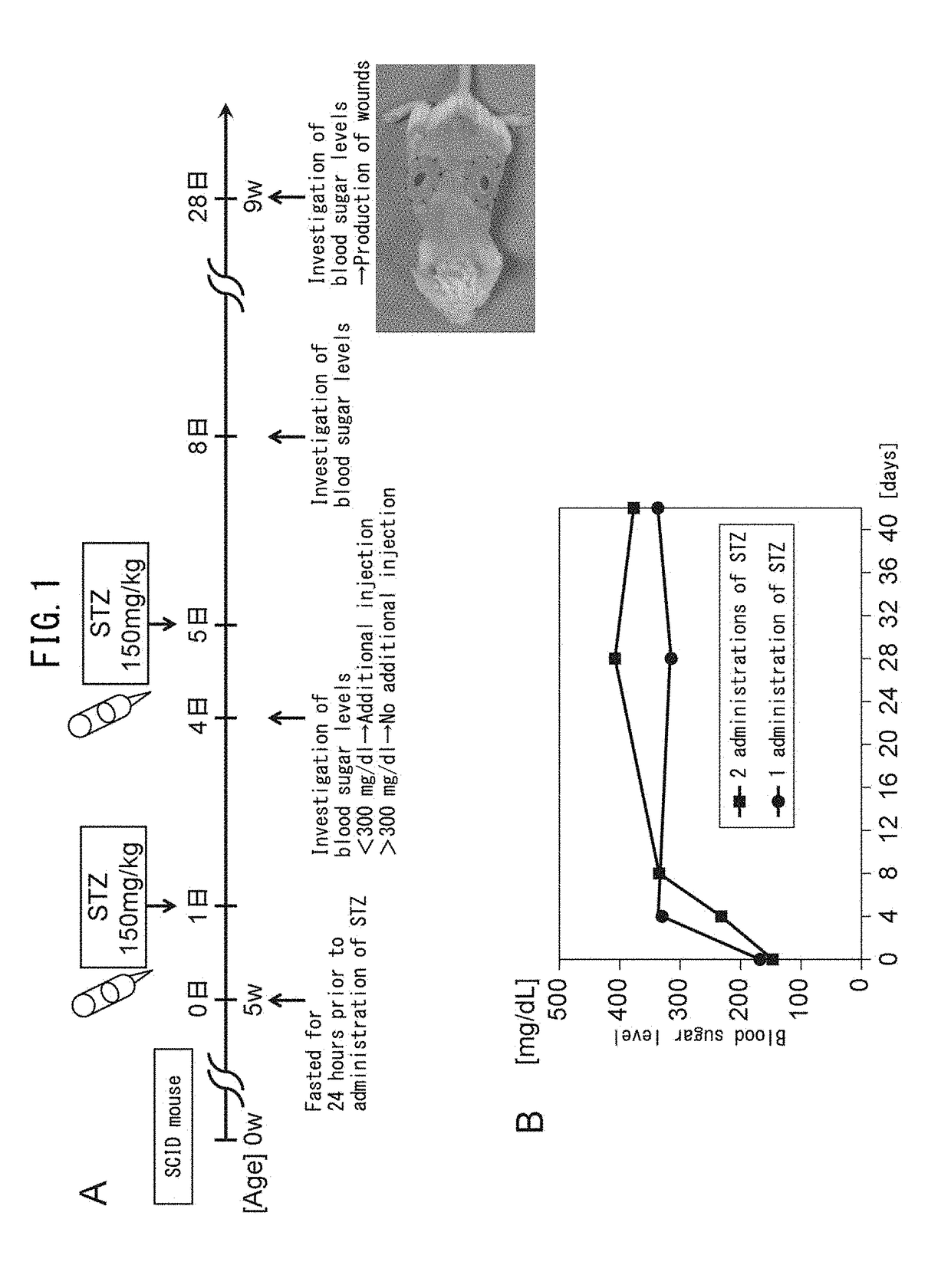
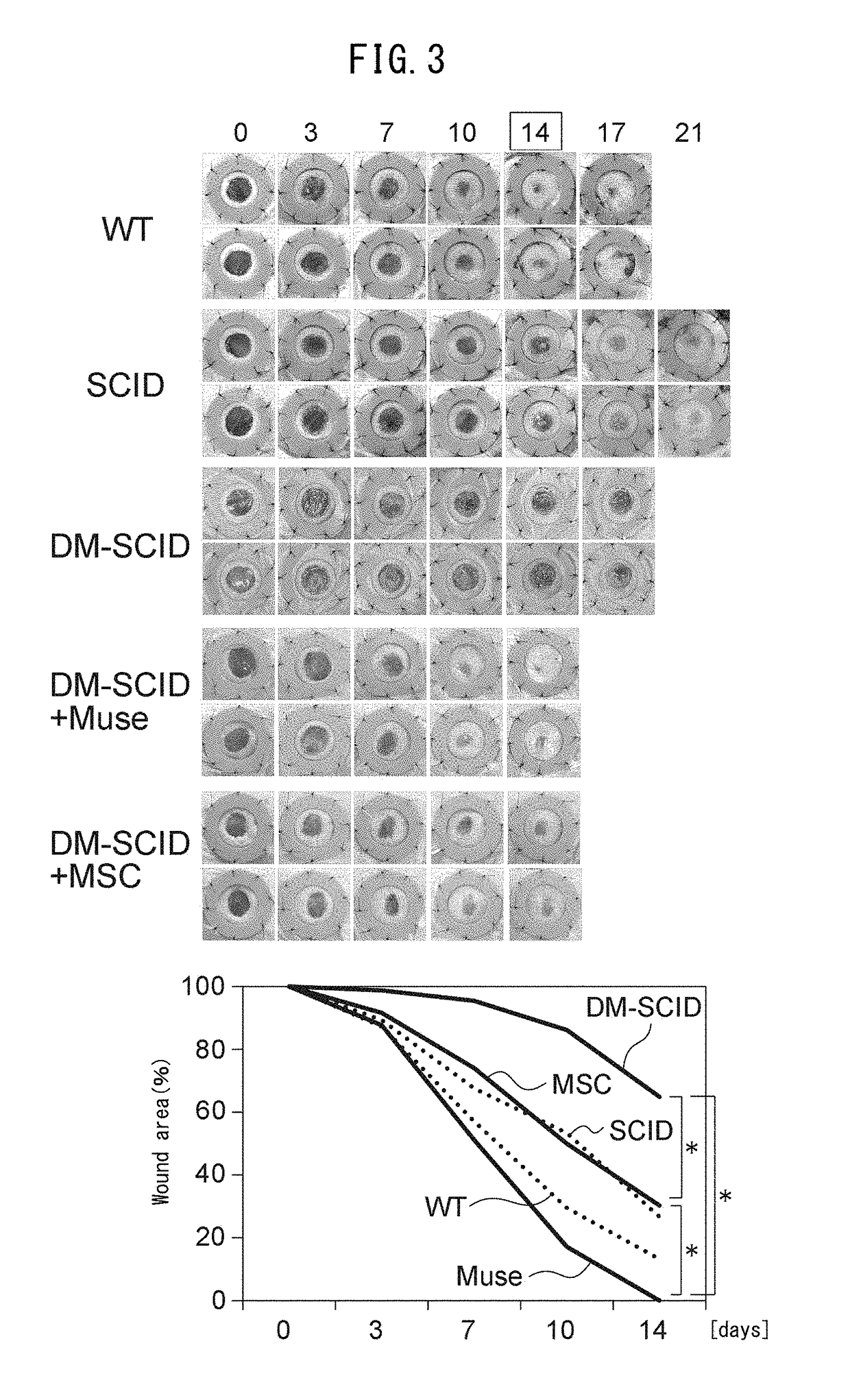
[0072]Five-week-old severe combined immunodeficient (SCID) mice (C. B17 / Icr-scid scid / scid) were purchased from Clea Japan, Inc. (Tokyo, Japan). All animal experiments were carried out with the approval of the Institutional Animal Care and Use Committee of the University of Tokyo. After allowing the SCID mice to fast for 24 hours, the animals were intraperitoneally injected with freshly prepared citrate-buffered saline (pH 4.5) containing streptozotocin (STZ, 150 mg / kg, Sigma-Aldrich, St. Louis Mo.). Blood glucose levels were measured using a glucometer and test strips (Glucose Pilot, Aventir Biotech LLC, Carlsbad, Calif.) on the third day after injection of STZ. Mice were considered to have diabetes mellitus (DM) if blood glucose level exceeded 300 mg / dl. Those mice that did not exhibit hyperglycemia (blood glucose level in excess of 300 mg / dl) were subjected to a second round of STZ injection (150 mg / kg) followed by monitoring bloo...
example 3
Cytokine Production Assay (ELISA)
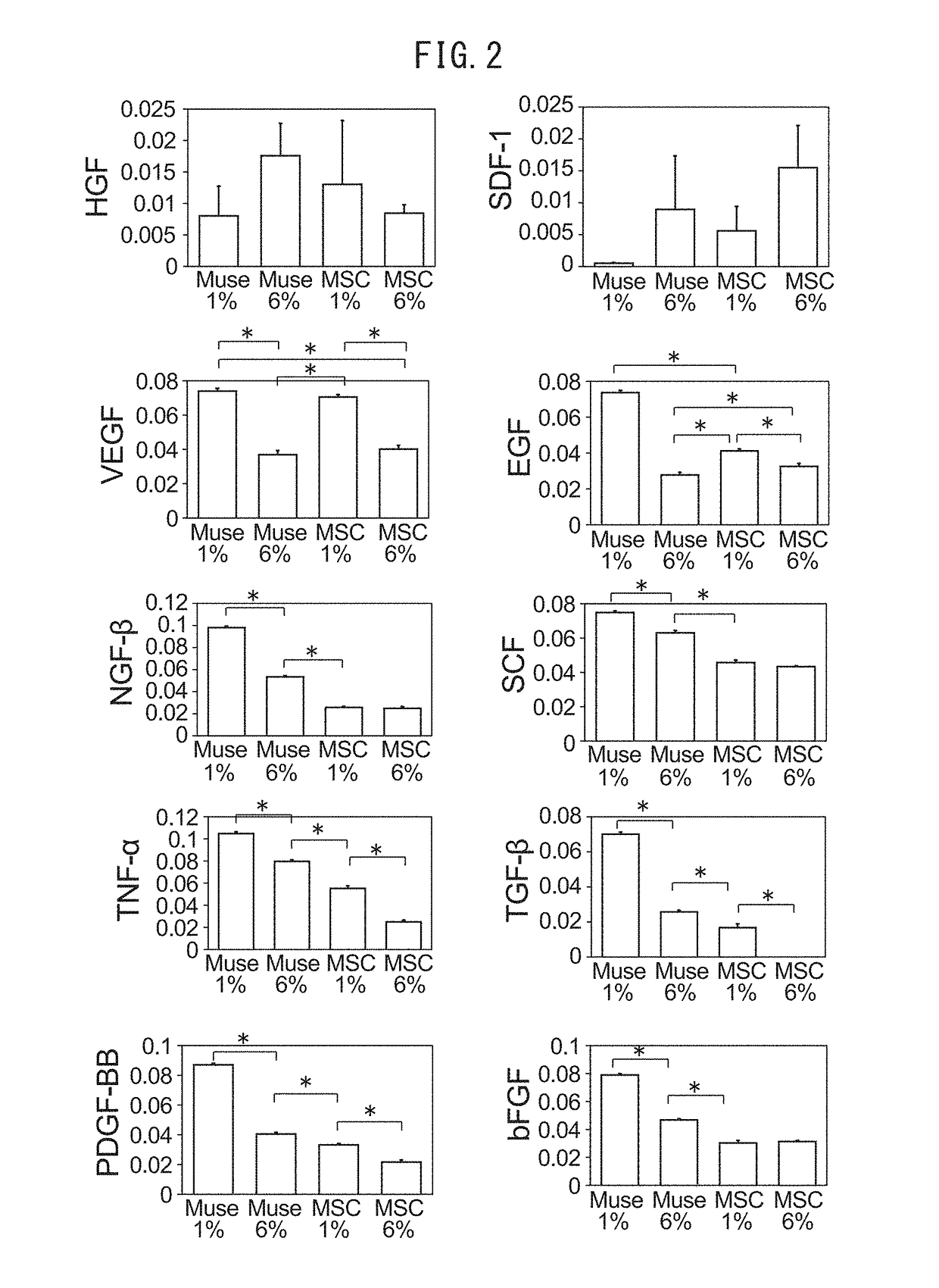
[0075]MSC are known to secrete growth factors (such as PDGF, bFGF, TGF-β or EGF) required in the inflammatory phase and cell growth phase of wound healing (Maxson, S., et al., Stem Cells Transl. Med., Vol. 1, p. 142-149 (2012)). Therefore, the Muse cell population and MSC were cultured in vitro under hypoxic and normoxic conditions to examine cytokines secreted into the culture broth. The experiment was carried out in the manner indicated below. 4.0×105 cells of the Muse cell population and MSC were disseminated in a 60 mm culture dish followed by culturing in serum-free DMEM under hypoxic (1% O2) and normoxic (6% O2) conditions. The culture medium was recovered 48 hours later and filtered using a 0.22 μm filter (Millex-GV Filter, Millipore, Billerica, Mass.). The amounts of cytokines secreted into the culture broth were compared and examined using an ELISA kit for hepatocyte growth factor (HGF) and stromal cell-derived factor 1 (SDF-1) (both availab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com