Amelioration and treatment of brain disorder resulting from fetal growth retardation using pluripotent stem cells
A technology for pluripotent stem cells and growth retardation, applied in the field of cell preparations to achieve the effect of reducing brain damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] (2) Preparation and use of cell preparations and pharmaceutical compositions
[0085]As far as the cell preparation and pharmaceutical composition of the present invention are concerned, there is no limitation, and the Muse cells obtained in the above (1) or the cell population containing Muse cells can be suspended in physiological saline, an appropriate buffer (such as phosphate-buffered saline) get in. In this case, when the number of Muse cells isolated from the tissue of oneself or another individual is small, the cells can be cultured and proliferated until a predetermined cell concentration is obtained before administration. It should be noted that, as reported (International Publication No. WO2011 / 007900 pamphlet), Muse cells do not become tumors, so even if they contain cells recovered from living tissue in an undifferentiated state, there is a possibility of canceration. Sex is also low and is safe. In addition, the recovered Muse cells can be cultured in a ...
Embodiment 1
[0103] Example 1: Production of fetal growth retardation model rats and administration of Muse cells
[0104] The experimental protocol involving experimental animals used in this study was approved by the Committee on Animal Experiments, Faculty of Medicine, Nagoya University. Pregnant SD rats were obtained from Japan SLC Co., Ltd. (Shizuoka Prefecture, Japan), put into cages where they could freely ingest food and water, and raised under a 12-hour light-dark cycle. Animal rooms and experimental spaces were maintained at 23°C at all times.
[0105] A vasoconstrictor (AC) (inner diameter: 0.45 mm or 0.40 mm) was installed at four positions in the uterine artery of a female rat on the 17th day of pregnancy to cause chronic ischemia of the fetus to create an intrauterine growth model. Rat pups produced by this female were used as models of fetal growth retardation. It should be noted that the fetal growth retardation of the rats born was determined by confirming the following ...
Embodiment 2
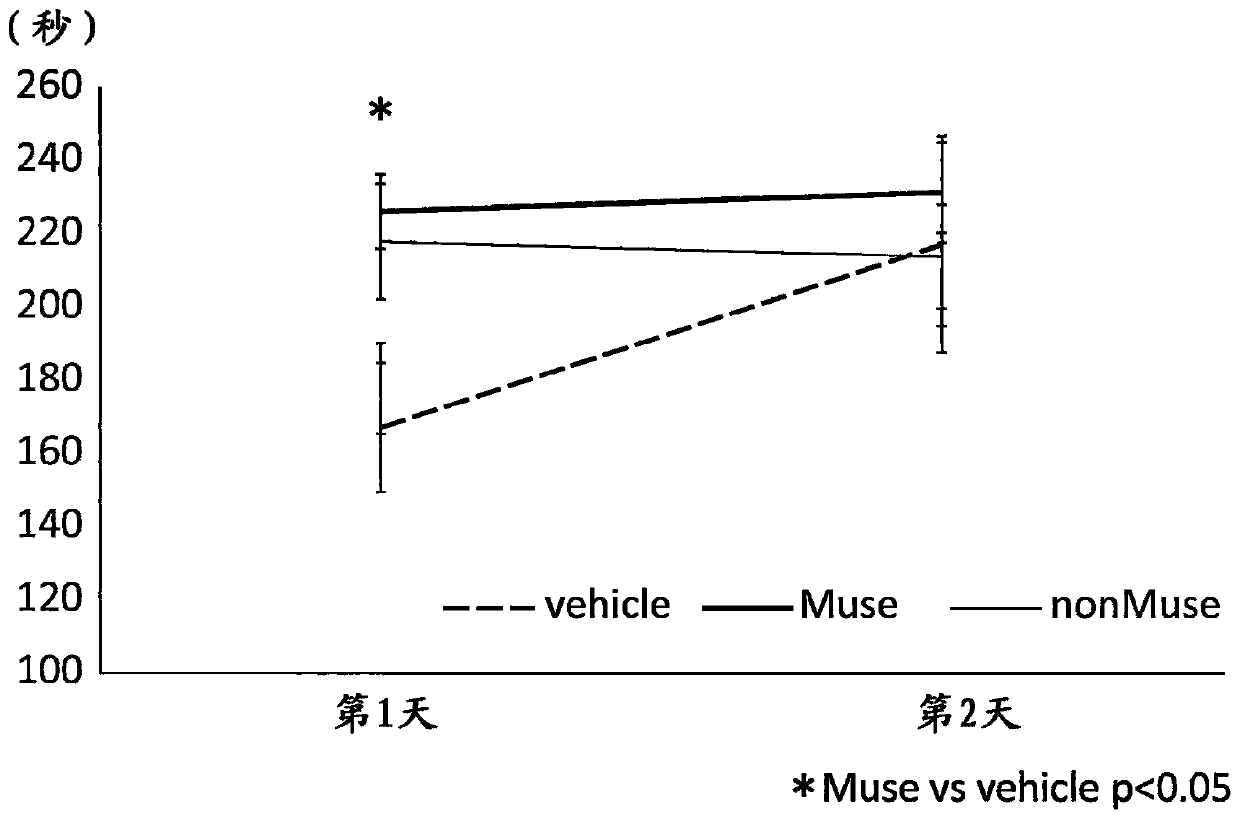
[0107] Example 2: Evaluation of brain function improvement (after one month after birth) using AC (0.45mm)
[0108] (1) Negative geotaxis test
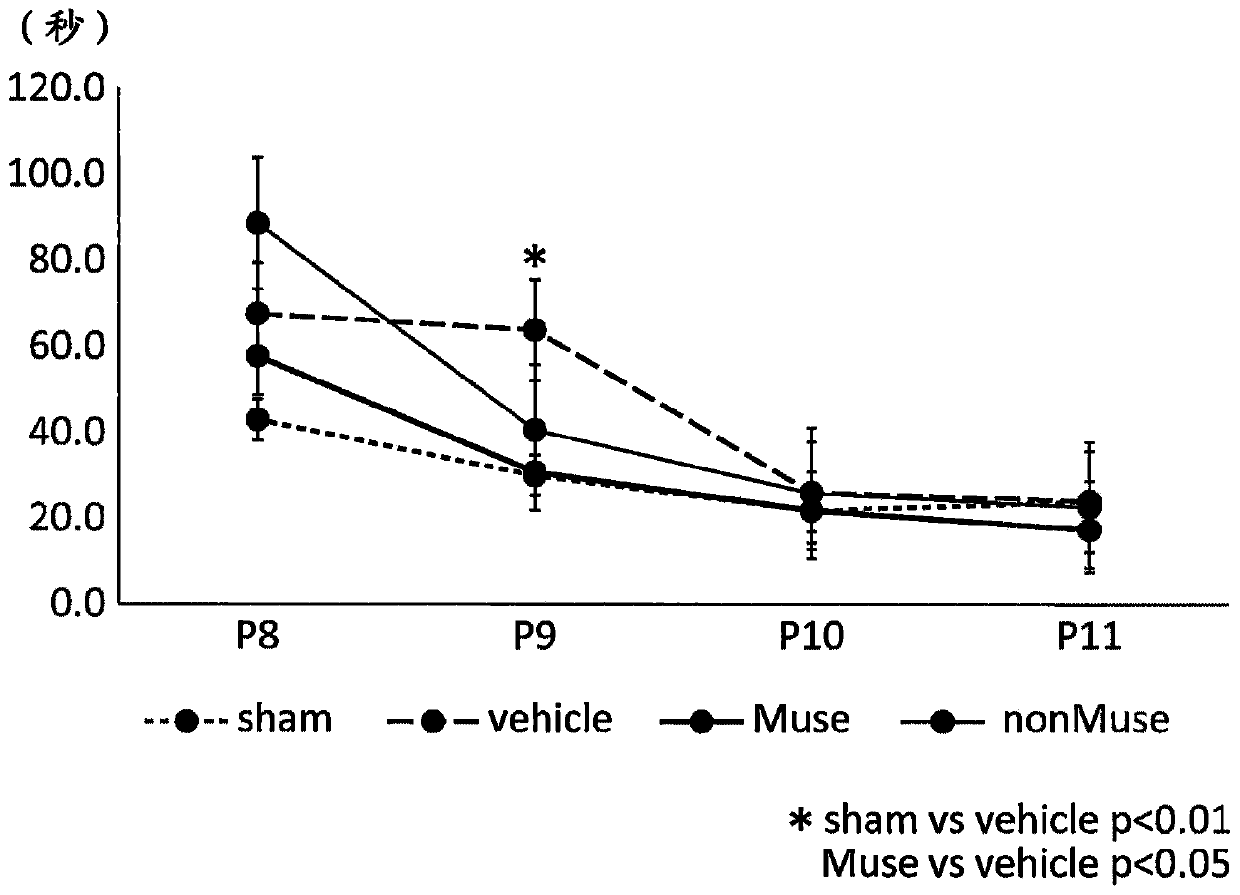
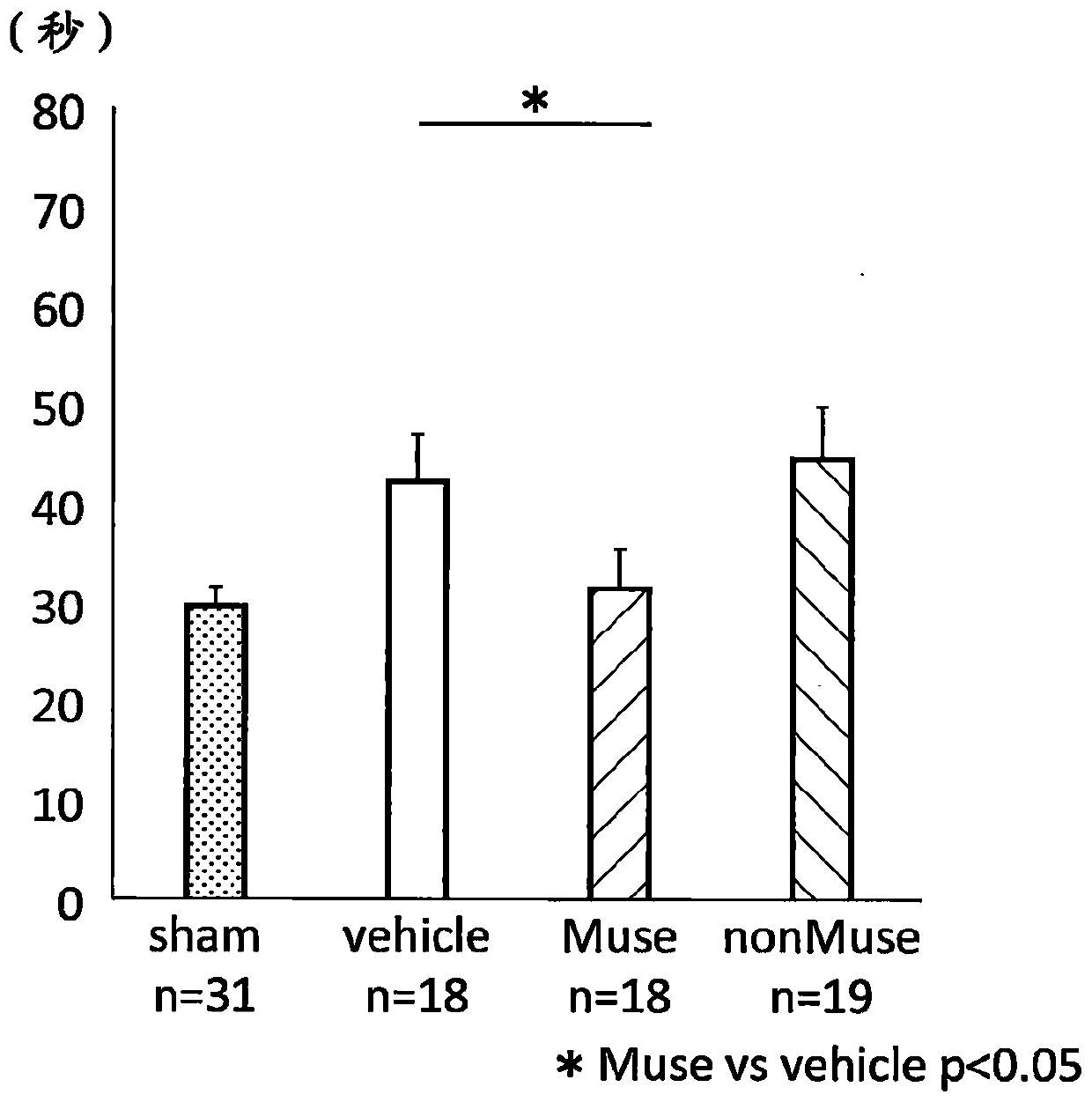
[0109] Improvement of behavioral disorder caused by brain damage accompanying fetal growth retardation by transplantation of Muse cells or the like was investigated by a negative geotaxis test. Fetal growth retardation model rats (hereinafter sometimes simply referred to as "rats") are placed head-down on a 30-degree inclined board, and the time (seconds) until the rat responds and climbs up is measured, and the time is used to Evaluate the effect of each treatment. The number of rats in each group was n=31 in the sham operation group, n=18 in the blank control group, n=19 in the Muse cell-administered group, and n=18 in the non-Muse cell group. In this test, rats were 8 to 11 days old, and the test was carried out once a day for 4 consecutive days. The negative geotaxis test of each group is shown in Figure 1A . The vertical axi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com