Alleviation and treatment of ischemia reperfusion-induced lung injury using pluripotent stem cells
A technology of pluripotent stem cells and ischemia-reperfusion, applied in the field of cell preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] (2) Preparation and use of cell preparations and pharmaceutical compositions
[0072] The cell preparation and pharmaceutical composition of the present invention are not limited, and are obtained by suspending the Muse cells or the cell population containing the Muse cells obtained in (1) above in physiological saline or an appropriate buffer (eg, phosphate-buffered physiological saline). In this case, when the number of Muse cells isolated from the autologous or allogeneic tissue is small, the cells are cultured and proliferated to obtain a predetermined cell concentration before administration. In addition, as has been reported (International Publication No. WO2011 / 007900), Muse cells do not become tumorigenic, and even if cells recovered from living tissue are contained in an undifferentiated state, the possibility of tumorigenicity is low, and it is safe. In addition, the culture of the recovered Muse cells is not particularly limited, and can be performed in a nor...
Embodiment 1
[0097] Example 1: Preparation of various cells
[0098] (1) Preparation of mesenchymal stem cells
[0099] Human mesenchymal stem cells (MSCs) purchased from Lonza Japan Co., Ltd. were used. As previously reported, using low glucose, L-glutamine-containing DMEM (Life Technologies, Carlsbad, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. , USA) and kanamycin medium, in 10cm culture dish at 37 ℃, 5% CO 2 conditions were cultivated. After the cells reached a cell density of 90% confluence, the cells were detached with 0.25% trypsin-EDTA (Biotech, Carlsbad, USA) and subcultured at a ratio of 1:2. The culture and passage were repeated in the same manner, and the cells of the 7th to 8th passage were used for the experiment.
[0100] (2) Preparation of Muse cells
[0101] The human MSCs were cultured and passaged, and the cells of the 7th to 8th passages were used. First, 5 ml of 5% bovine serum albumin (Sigma-Aldrich, St. Louis, USA) and 1 ml of 100 mM EDT...
Embodiment 2
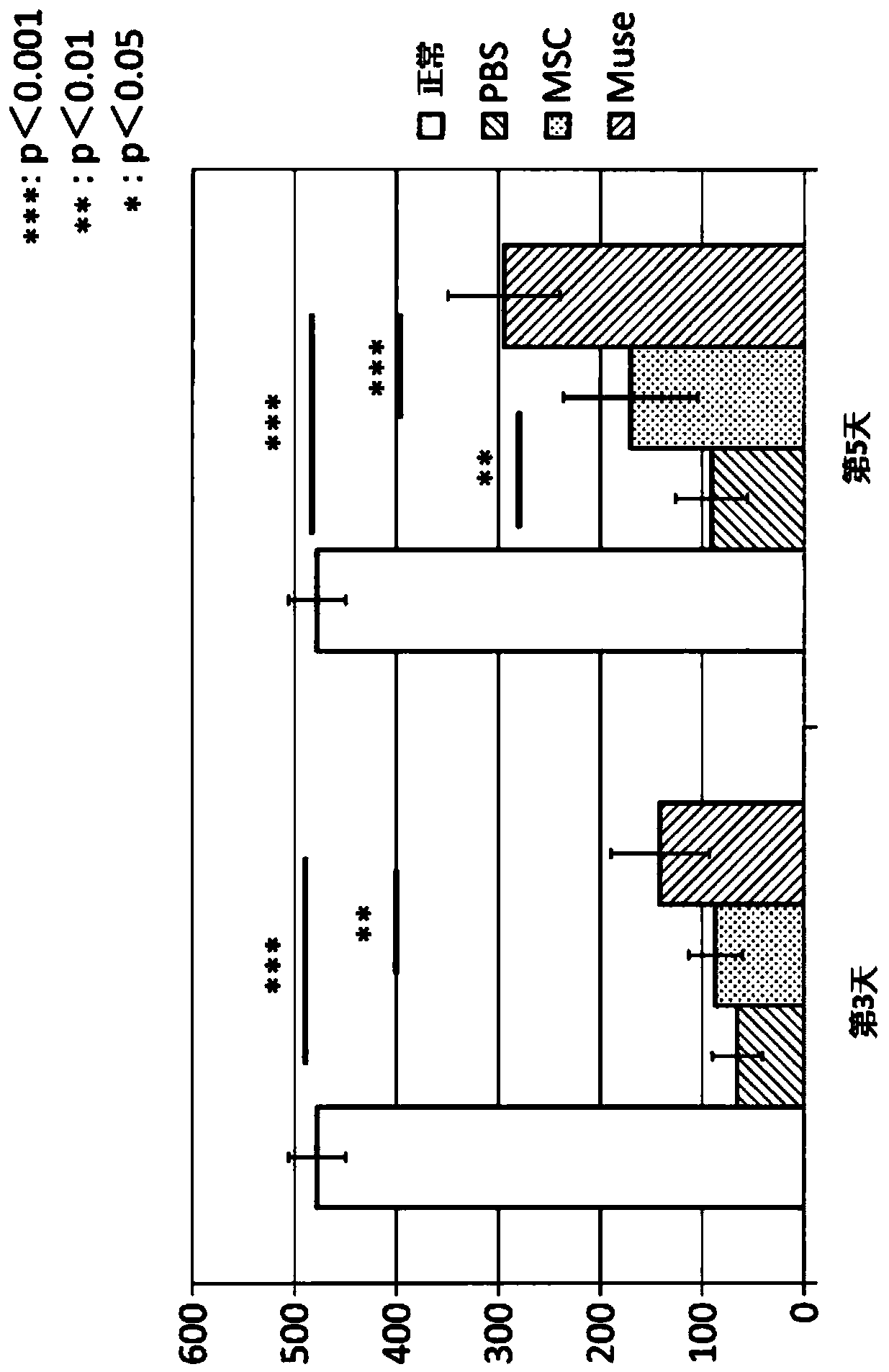
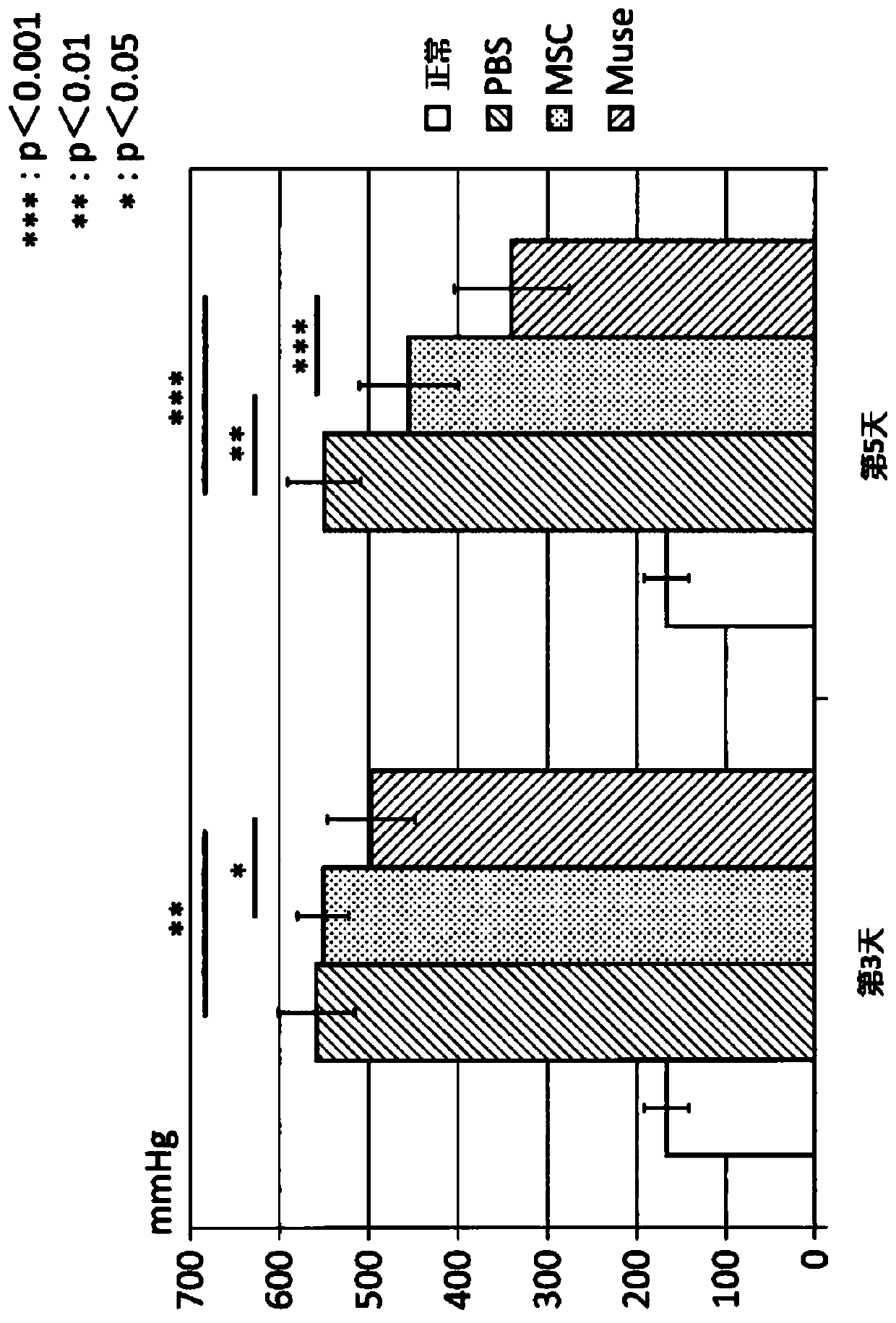
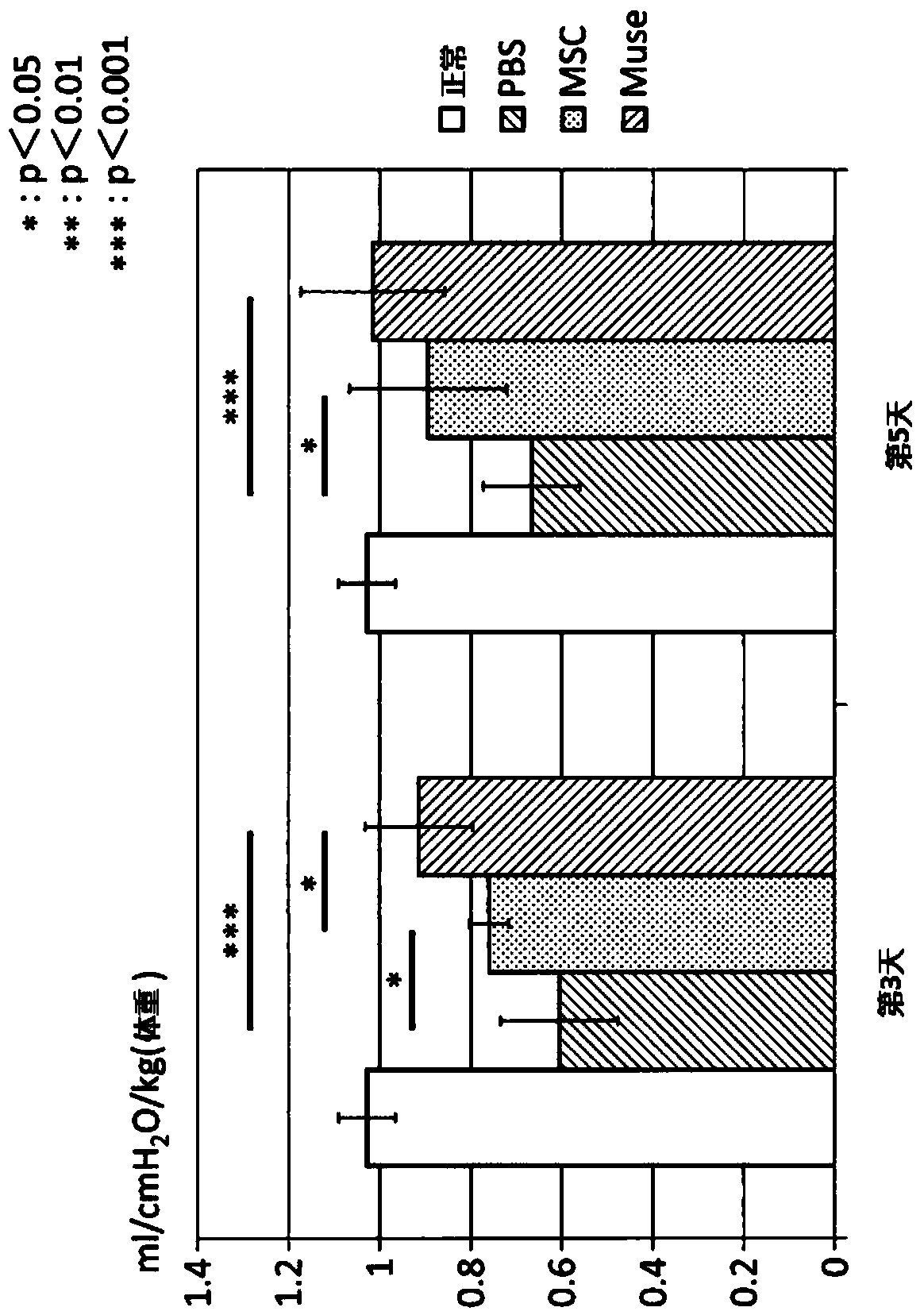
[0102] Example 2: Preparation of ischemia-reperfusion lung injury model rats and administration of various cells
[0103] In this study, a total of four groups were set up: the PBS group administered with PBS to ischemia-reperfusion lung injury model rats, the MSC group administered with human MSCs, the Muse group administered with Muse cells, and the normal group without surgery. Evaluation of lung function after ischemia-reperfusion lung injury was performed with n=8 for each group. Just after reperfusion, the left pulmonary artery was punctured with a 30G needle, and 200 μl of PBS was given to the PBS group for 1 minute, and 1.5×10 5 Cell MSC / 200μl PBS, give 1.5×10 to Muse group 5 Cell Muse cells / 200μl PBS.
[0104] Male Sprague-Dawley rats born at 9 weeks old and weighing 290 to 340 g were used. Anesthetize by inhalation of isoflurane. After obtaining a sufficiently quiet state, endotracheal intubation was performed through a 14G vascular catheter, and an artificial ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com