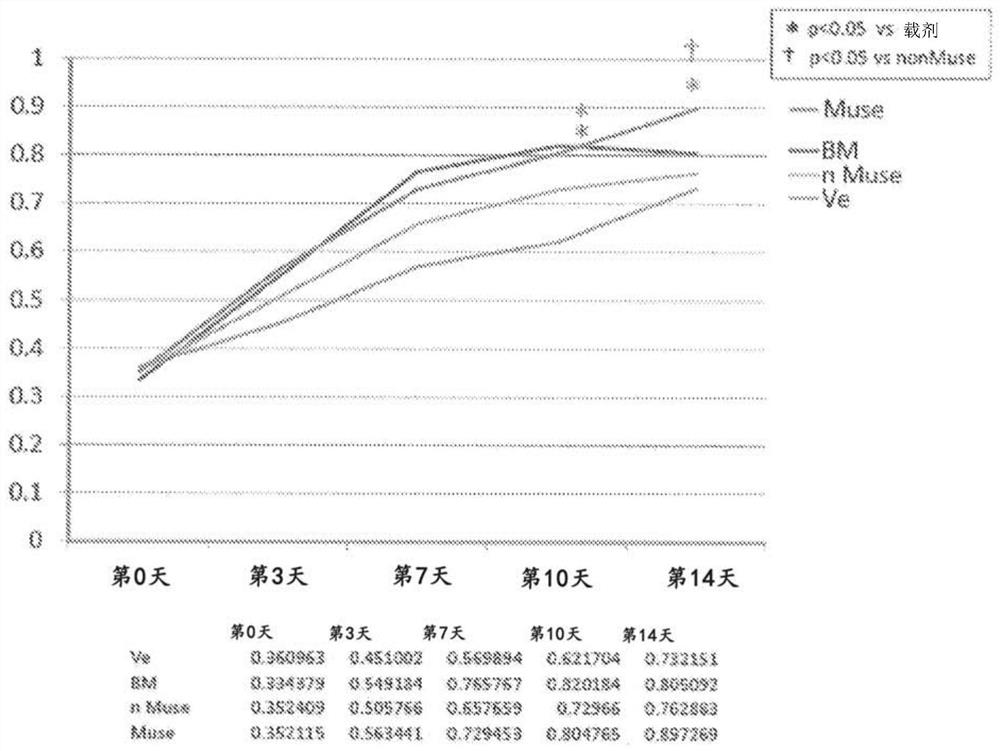
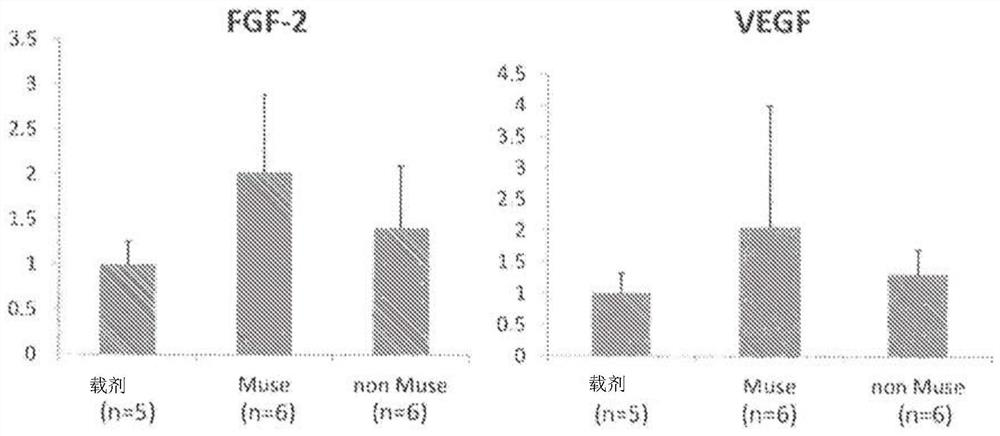
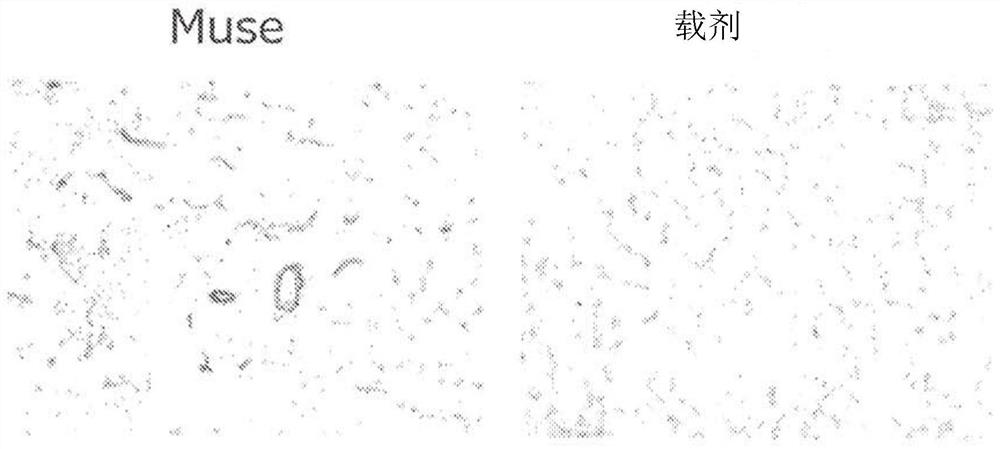
Therapeutic agent of peripheral blood flow disorder
A peripheral blood and disorder technology, applied in the field of cell preparations of pluripotent stem cells, can solve the problem of using Muse cells, etc., and achieve the effect of improving or restoring blood flow disorder and excellent safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0096] (2) Preparation and use of cell preparations
[0097] The cell preparation of the present invention is not particularly limited, and can be obtained by suspending the Muse cells obtained in (1) above or a cell population containing Muse cells in physiological saline or an appropriate buffer (for example, phosphate-buffered saline). In this case, if the number of Muse cells isolated from autologous or allogeneic tissues is small, they can be cultured before cell transplantation until they proliferate to obtain a predetermined number of cells. In addition, as reported (International Publication No. WO2011 / 007900 pamphlet), since Muse cells do not become tumorous, even if the cells recovered from living tissue contain an undifferentiated state, there is little concern about their canceration, So it's safe. In addition, culture of the recovered Muse cells is not particularly limited, and can be performed in an ordinary growth medium (for example, α-minimal essential medium...
Embodiment 1
[0104] Example 1: Preparation of lower limb ischemia model mice
[0105] The experimental protocol using mice in this example complied with the "Kyoto Prefectural University of Medicine Animal Experiments, etc. Relevant Rules", and experimental animals were prepared under the supervision of the Animal Experiment Center of Kyoto Prefectural University of Medicine in accordance with the rules. More specifically, it is prepared by the following steps.
[0106] Female BALB / C nude mice aged 8-10 weeks were anesthetized (induction: 4%, maintenance: 2%) by isoflurane inhalation. The lower limbs were cut open, and the periphery of the left common femoral artery and vein and the left superficial femoral artery and vein were ligated with 5-0 silk thread under a stereomicroscope (KONAN OPERATION MICROSCOPE KOM). The mice thus prepared were used as lower limb ischemia model mice in the following experiments.
Embodiment 2
[0107] Embodiment 2: the preparation of human Muse cell
[0108] Muse cells were obtained according to the method described in International Publication No. WO 2011 / 007900 concerning the isolation and identification of human Muse cells. Commercially available mesenchymal stem cells (MSC, Lonza Corporation) were used as a source of Muse cells. The Muse cells used for transplantation expressed green fluorescent protein (GFP) to confirm that they had attached to the damaged peripheral arterial site, and the lentiviral GFP gene was introduced into the Muse cells in advance to label the cells. GFP-labeled Muse cells were isolated by FACS as double positive cells for GFP and SSEA-3. In addition, the remaining cells from which Muse cells were isolated from MSCs were used as non-Muse cells. In addition, GFP-positive MSCs were also isolated by FACS and used as an MSC panel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com