Compound with antitumor effect and its preparation method and application
A technology of anti-tumor effects and compounds, applied in the direction of anti-tumor drugs, rhodium organic compounds, chemical instruments and methods, etc., can solve the problems of reducing the anti-tumor effects of nucleoside drugs, mutation of tumor cell lines, strong toxic side effects, etc., to achieve Good biological activity and efficacy, less toxic and side effects, and stable molecular structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
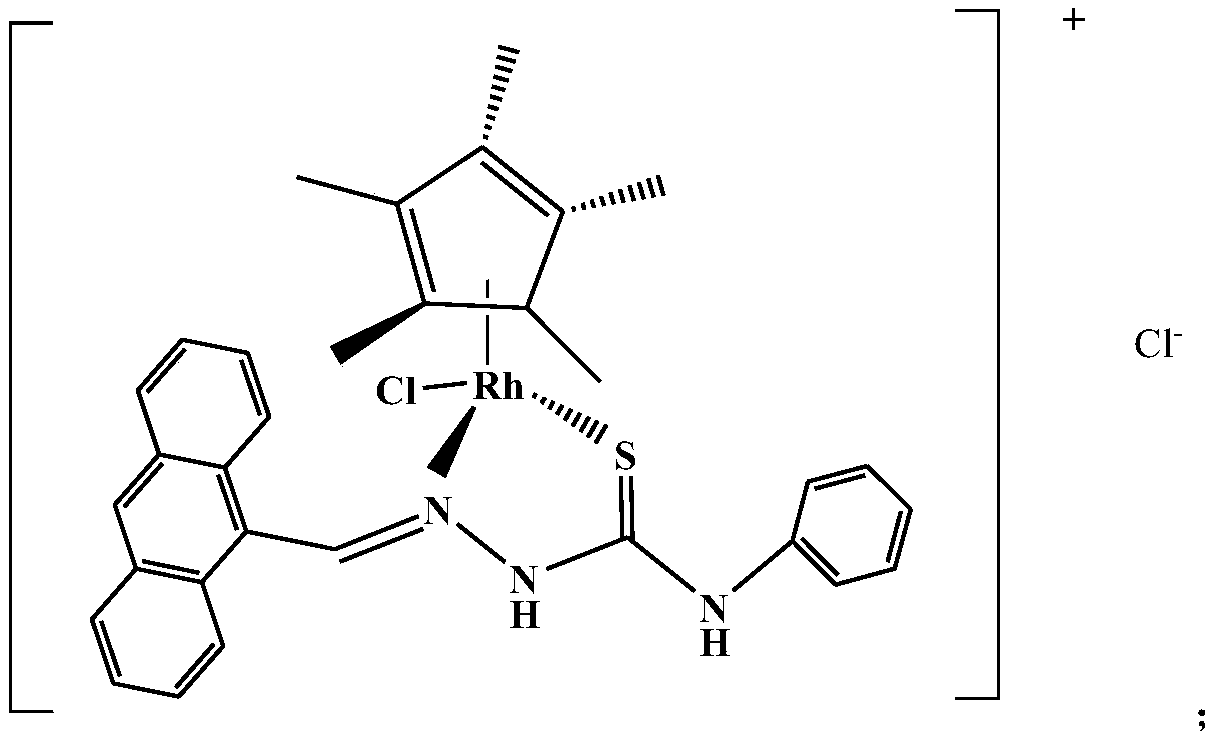
[0032] A compound with anti-tumor effect, the structural formula of the compound is:
[0033]
[0034] A preparation method of a compound with anti-tumor effect, comprising the following steps:
[0035] S1. Dissolve 9-anthracene formaldehyde and 4-phenyl-3-thiosemicarbazide in absolute ethanol, then add glacial acetic acid, heat to reflux, cool and filter to obtain 9-anthracene-N 4 - phenylthiosemicarbazide;
[0036] S2, the 9-anthracene-N 4 - Phenylthiosemicarbazide and dichloro(pentamethylcyclopentadienyl) rhodium (III) dimer are dissolved in ethanol and mixed, and the solvent is removed by rotary evaporation to obtain a red solid, which is the compound of the anti-tumor effect .
[0037] In the preparation method of the compound with anti-tumor effect, the heating and reflux time in S1 is 3 hours.
[0038] In the preparation method of the compound with anti-tumor effect, the mixing temperature in S2 is 15° C. and the mixing time is 3 hours.
[0039] In the preparati...
Embodiment 2
[0043] A preparation method of a compound with anti-tumor effect, comprising the following steps:
[0044] S1. Dissolve 9-anthracene formaldehyde and 4-phenyl-3-thiosemicarbazide in absolute ethanol, then add glacial acetic acid, heat to reflux, cool and filter to obtain 9-anthracene-N 4 - phenylthiosemicarbazide;
[0045] S2, the 9-anthracene-N 4 - Phenylthiosemicarbazide and dichloro(pentamethylcyclopentadienyl) rhodium (III) dimer are dissolved in ethanol and mixed, and the solvent is removed by rotary evaporation to obtain a red solid, which is the compound of the anti-tumor effect .
[0046] In the preparation method of the compound with anti-tumor effect, the heating and reflux time in S1 is 4 hours.
[0047] In the preparation method of the compound with anti-tumor effect, the mixing temperature in S2 is 25° C. and the mixing time is 4 hours.
[0048] In the preparation method of the anti-tumor compound, S2 also includes recrystallizing the obtained red solid in dic...
Embodiment 3
[0052] A preparation method of a compound with anti-tumor effect, comprising the following steps:
[0053] S1. Dissolve 9-anthracene formaldehyde and 4-phenyl-3-thiosemicarbazide in absolute ethanol, then add glacial acetic acid, heat to reflux, cool and filter to obtain 9-anthracene-N 4 - phenylthiosemicarbazide;
[0054] S2, the 9-anthracene-N 4 - Phenylthiosemicarbazide and dichloro(pentamethylcyclopentadienyl) rhodium (III) dimer are dissolved in ethanol and mixed, and the solvent is removed by rotary evaporation to obtain a red solid, which is the compound of the anti-tumor effect .
[0055] In the preparation method of the compound with anti-tumor effect, the heating and reflux time in S1 is 5 hours.
[0056] In the preparation method of the compound with anti-tumor effect, the mixing temperature in S2 is 30° C. and the mixing time is 6 hours.
[0057] In the preparation method of the anti-tumor compound, S2 also includes recrystallizing the obtained red solid in dic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com