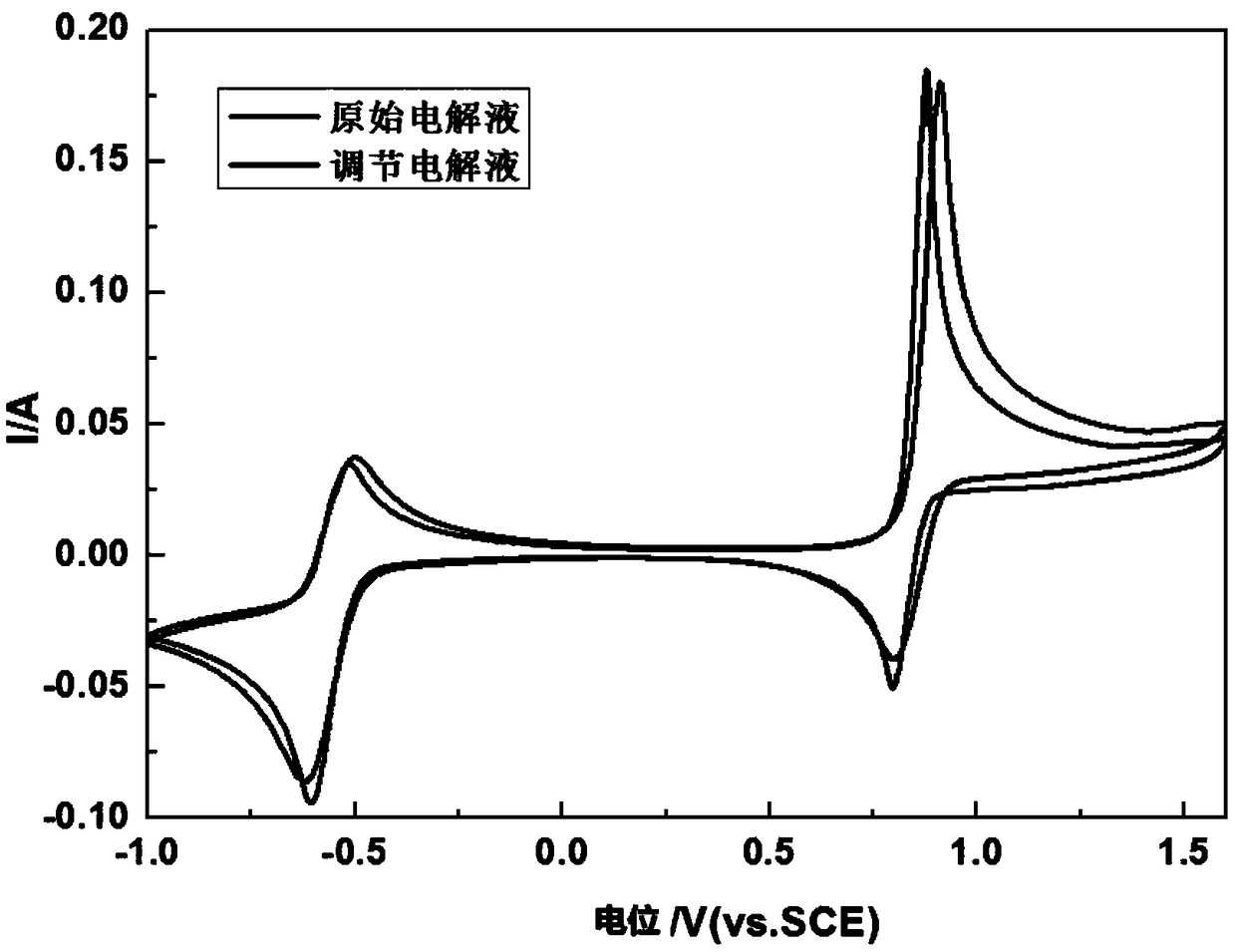
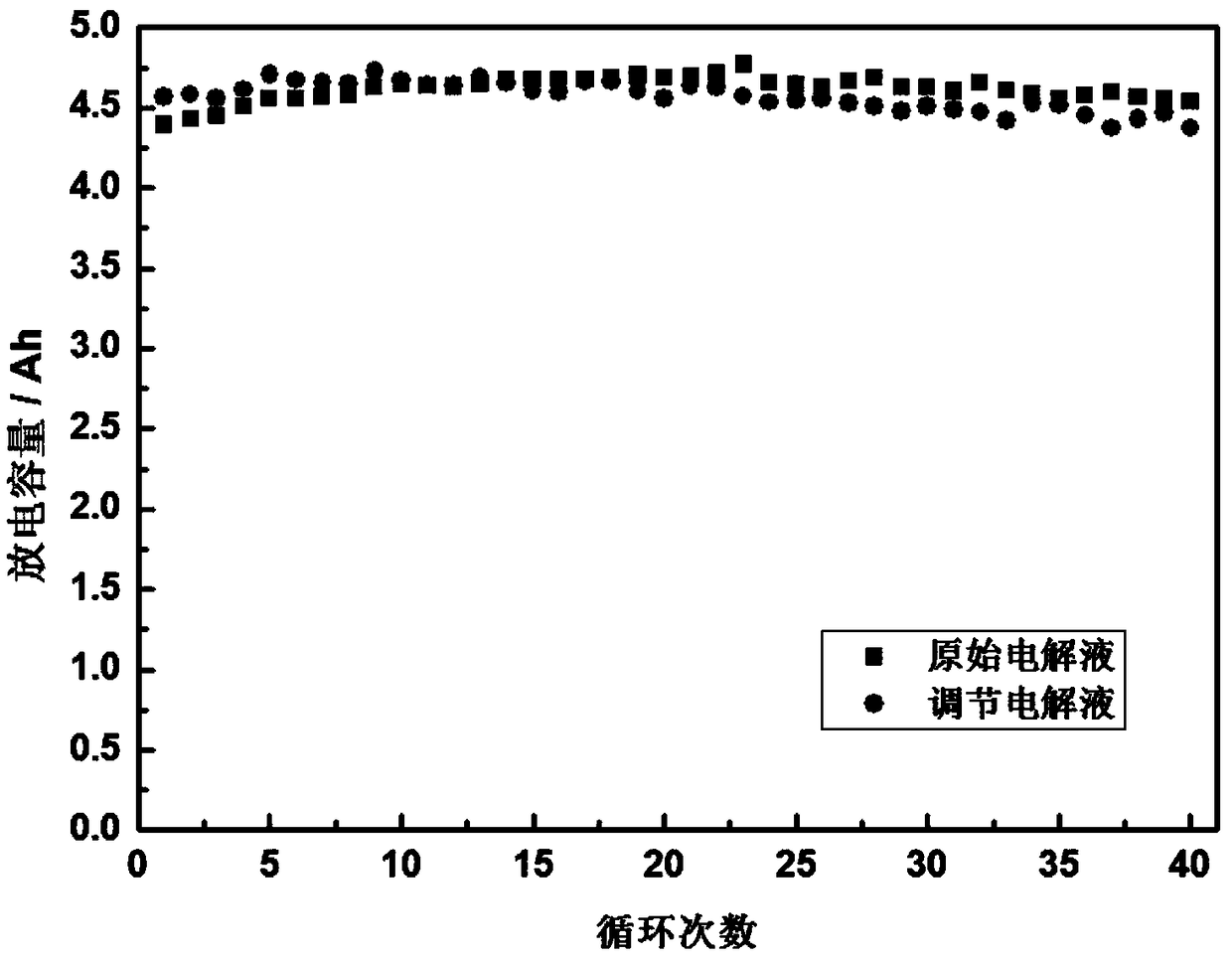
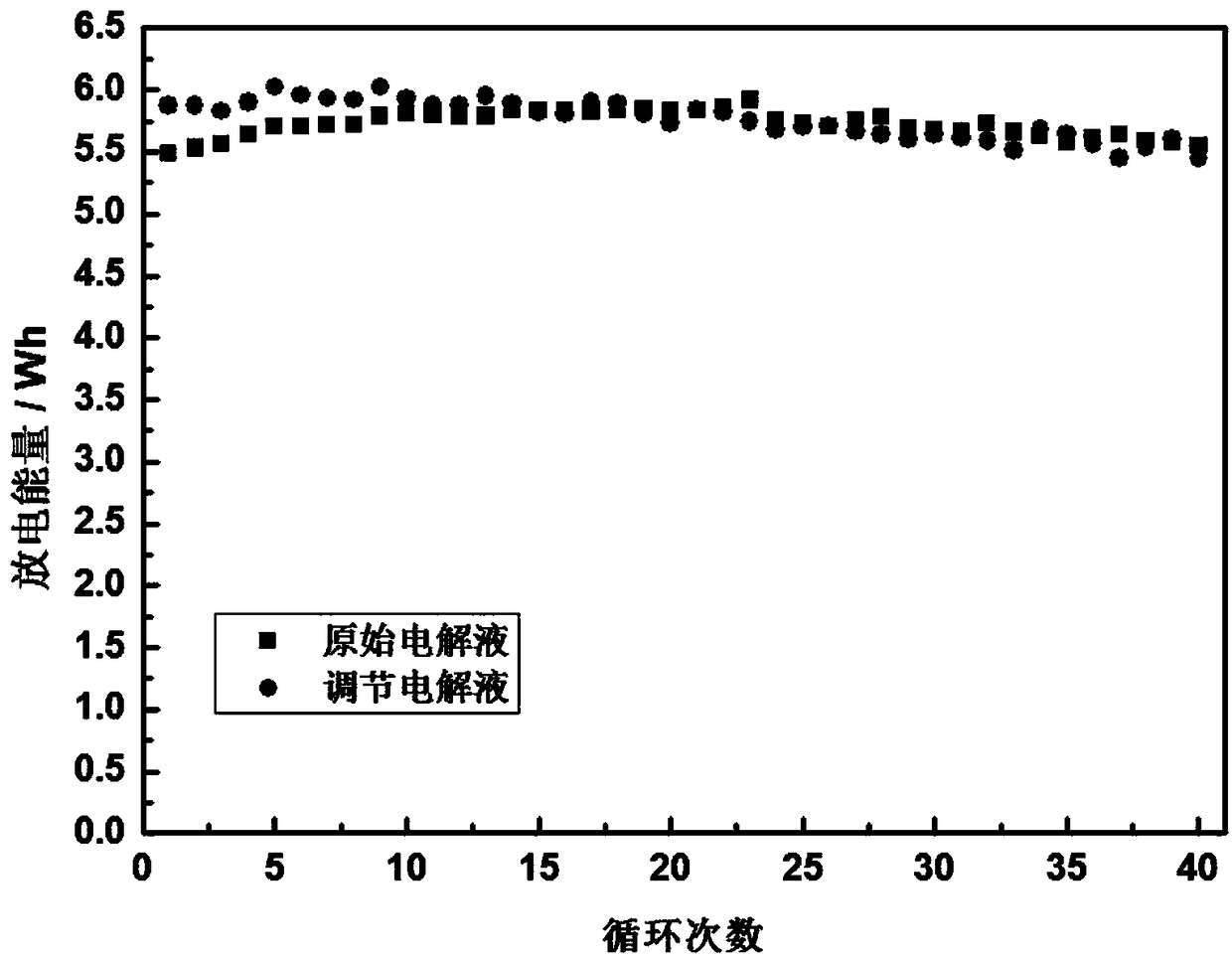
Method of regulating the valence of vanadium in electrolyte of vanadium cell
An electrolyte and vanadium battery technology, applied in the field of vanadium batteries, can solve the problems of increased energy storage costs and replacement of vanadium batteries, and achieve the effects of avoiding waste, simple and easy operation, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 uses vanadium dichloride to adjust the vanadium valence state of the electrolyte of vanadium battery
[0042] Using chemical titration, the concentration of vanadium ions in the vanadium electrolyte that needs to be adjusted to the valence state of vanadium ions is 1.5mol / L, and the average valence is +4.4; the concentration of chloride ions in the vanadium electrolyte is 8.6mol / L; The volume of the electrolytic solution was 20.0 L. The vanadium ion concentration of the target vanadium electrolyte is 1.6mol / L, the average valence state is +3.5, and the chloride ion concentration is 8.8mol / L. The only compound of vanadium is vanadium dichloride. Therefore, according to formula (1), it can be known that the amount of substance n required to obtain vanadium dichloride 5 is 18.0mol, calculate the volume V of the target electrolyte according to the formula (2) 2 is 30.0L, and the amount of required HCl is calculated according to formula (3) to be 28.0mol. Fi...
Embodiment 2
[0045] Example 2 Using vanadium trichloride to adjust the vanadium valence state of the vanadium battery electrolyte
[0046] Using chemical titration, the concentration of vanadium ions in the vanadium electrolyte that needs to be adjusted to the valence state of vanadium ions is 1.5mol / L, and the average valence is +4.4; the concentration of chloride ions in the vanadium electrolyte is 8.6mol / L; The volume of the electrolytic solution was 20.0 L. The vanadium ion concentration of the target vanadium electrolyte is 1.6mol / L, the average valence state is +3.5, and the chloride ion concentration is 8.8mol / L. The only compound of vanadium is vanadium trichloride. Therefore, according to formula (1), it can be known that the amount of substance n required to obtain vanadium trichloride 4 is 54.0mol, calculate the volume V of the target electrolyte according to the formula (2) 2 is 52.5L, and the amount of required HCl substance calculated according to formula (3) is 128.0mol. ...
Embodiment 3
[0047] Example 3 Using vanadium trioxide to adjust the vanadium valence state of the vanadium battery electrolyte
[0048] Using chemical titration, the concentration of vanadium ions in the vanadium electrolyte that needs to be adjusted to the valence state of vanadium ions is 1.5mol / L, and the average valence is +4.4; the concentration of chloride ions in the vanadium electrolyte is 8.6mol / L; The volume of the electrolytic solution was 20.0 L. The vanadium ion concentration of the target vanadium electrolyte is 1.6mol / L, the average valence state is +3.5, and the chloride ion concentration is 8.8mol / L. The only compound of vanadium is vanadium trioxide. Therefore, according to formula (1), it can be known that the amount of substance n required to obtain the required vanadium trioxide 3 is 27.0mol, calculate the volume V of the target electrolyte according to the formula (2) 2 is 52.5L, and the amount of required HCl substance calculated according to formula (3) is 290.0m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com