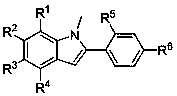
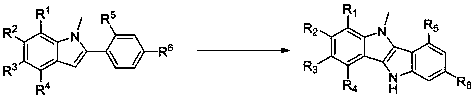
Fused bisindole derivatives and preparation method thereof
A technology for fused bisindole derivatives and indole, which is applied in the field of preparation of organic compounds, can solve the problems of harsh reaction conditions, high risk, and large pollution, and achieve easy availability of raw materials, various types, reduced pollution and production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: 5-methyl-5,10-dihydroindoline[3,2- b ] Synthesis of indole
[0034] With 1-methyl-2-phenylindole as raw material, the reaction steps are as follows:
[0035] Add 1-methyl-2-phenylindole (0.21 g, 1 mmol), sodium nitrite (0.14 g, 2 mmol), potassium peroxodisulfate (0.54 g, 2 mmol) and acetone (5 mL ), nitrosation reaction was carried out at 60°C; TLC followed the reaction until it was completely completed; sampling was carried out for NMR, the yield was 95%, and the analysis data was as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 8.30 (dd, J = 5.7, 2.5 Hz, 1H), 7.91 – 7.79 (m, 2H), 7.66 – 7.56 (m, 3H), 7.49 –7.34 (m, 3H), 3.87 (s, 3H);
[0036] After the nitroso reaction, acetone was removed by rotary evaporation, and then triethylphosphite (0.83 g, 5 mmol) was added to the bottle, and the ring-forming reaction was carried out at 110 °C;
[0037] TLC followed the reaction until it was completely finished; the crude product obtained after the reaction w...
Embodiment 2
[0038] Example 2: Synthesis of 3-chloro-10-methyl-5,10-dihydroindo[3,2-b]indole
[0039]
[0040] Add 1-methyl-2-phenyl-5-chloroindole (0.24 g, 1 mmol), sodium nitrite (0.21 g, 3 mmol), potassium peroxodisulfate (0.54 g, 2 mmol) to a reaction vial and acetonitrile (5 milliliters), nitrosation reaction was carried out at 70°C; TLC tracked the reaction until it was completely completed; sampling was carried out for NMR, the yield was 96%, and the analysis data was as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 8.22 (d, J = 1.7 Hz, 1H), 7.85 – 7.74 (m, 2H), 7.64 – 7.53 (m, 3H), 7.34 –7.23 (m, 2H), 3.83 (s, 3H);
[0041] After the nitrosation reaction, acetonitrile was removed by rotary evaporation, then triethylphosphite (0.83 g, 5 mmol) was added to the bottle, and the ring-forming reaction was carried out at 110 °C;
[0042] TLC followed the reaction until it was completely completed; the crude product obtained after the reaction was separated by column chromatography ...
Embodiment 3
[0043] Example 3: Synthesis of 3-nitro-10-methyl-5,10-dihydroindo[3,2-b]indole
[0044] Add 1-methyl-2-phenyl-5-nitroindole (0.25 g, 1 mmol), sodium nitrite (0.21 g, 3 mmol), potassium peroxodisulfate (0.27 g, 1 mmol) to the reaction flask ) and dimethyl sulfoxide (5 ml), nitrosation reaction was carried out at 100°C; TLC followed the reaction until it was completely completed; sampling was carried out for NMR, the yield was 92%, and the analysis data was as follows: 1 H NMR (400 MHz, DMSO- d 6 ): δ 8.77 (m, 1H), 8.31 (m, 1H), 8.00 (m, 3H), 7.79 – 7.66 (m, 3H),3.95 (s, 3H).
[0045] After the nitrosation reaction, dimethyl sulfoxide was removed by rotary evaporation, then triethyl phosphite (0.83 g, 5 mmol) was added to the bottle, and the ring formation reaction was carried out at 120 °C;
[0046] TLC followed the reaction until it was completely over; the crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl aceta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com