Subcutaneous embedding thread for treating epilepsy
A technology of implanting thread and epilepsy, which is applied in the field of biopharmaceuticals, can solve the problems of large side effects, systemic and gastrointestinal reactions, liver and kidney function damage, etc., and achieve the effects of slow degradation, less surgical pain, and long use time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
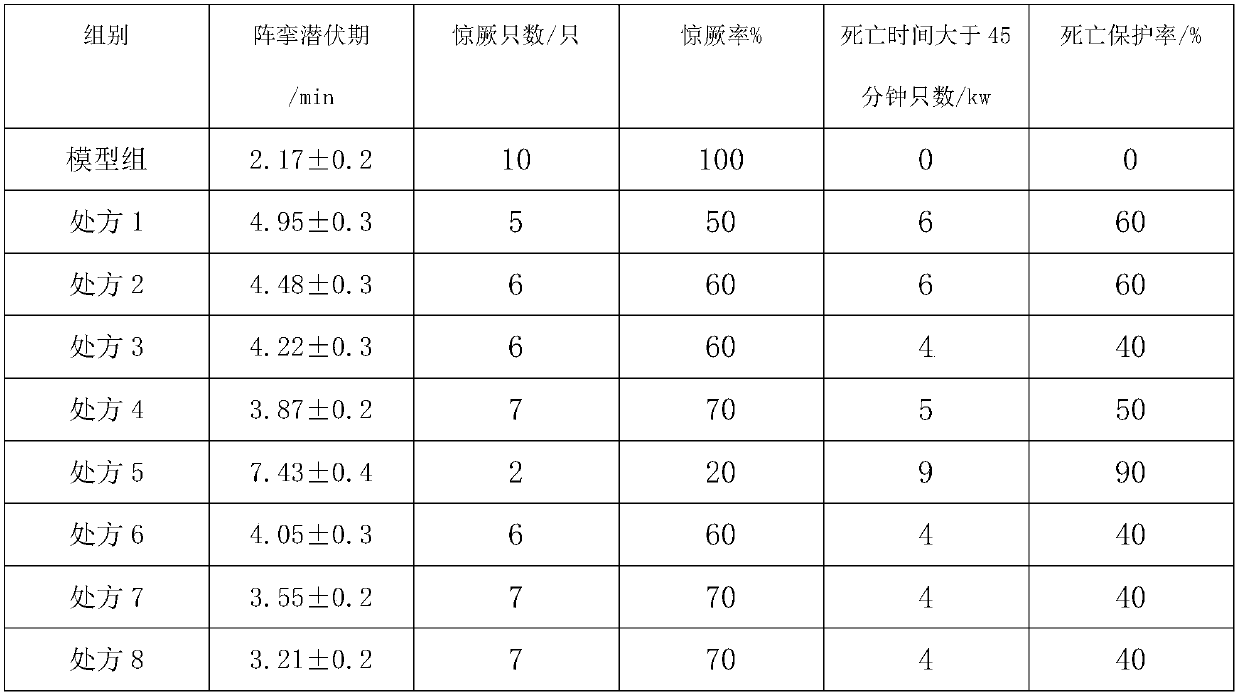
Embodiment 1
[0122] API:
[0123] Phenytoin 2.4kg, carbamazepine 1.2kg, lamotrigine 0.6kg;
[0124] Accessories:
[0125] Frozen phenytoin, carbamazepine and lamotrigine respectively at -15°C for 2 hours and then ultrafinely pulverized with a superfine pulverizer to obtain superfine powder; spray 0.5kg of dry ice as a coolant during the superfine pulverization process ; At least 90% of the micropowder particle size in the superfine pulverization is not more than 5 microns; superfine pulverization to obtain ultrafine powder for later use; put polylactic acid and polyethylene glycol into the reaction kettle, and heat at 100 ° C, while heating Stir for 3 hours, then add phenytoin sodium superfine powder and continue to stir for 0.5 hours. When the auxiliary materials in the reaction kettle are in a viscous state, add methylcellulose sodium and continue to stir for 1 hour, and finally add carbamazepine and Lamosan Oxyzine superfine powder, stir evenly to obtain a mixture, cool and air-dry th...
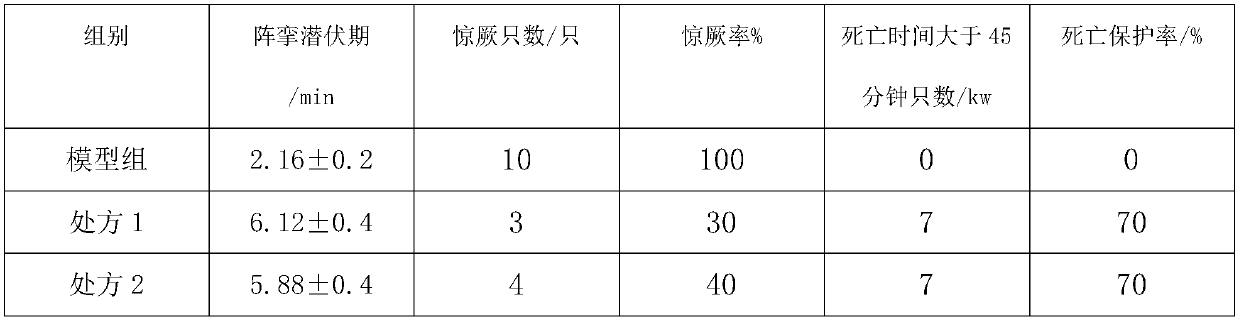
Embodiment 2
[0127] API:
[0128] API:
[0129] Phenytoin 1.5kg, carbamazepine 1.5kg, lamotrigine 0.5kg;
[0130] Accessories:
[0131] Sodium methylcellulose 0.5kg, polylactic acid 0.15kg, polyethylene glycol 0.25kg.
[0132] Frozen phenytoin, carbamazepine and lamotrigine respectively at -15°C for 2 hours and then ultrafinely pulverized with a superfine pulverizer to obtain superfine powder; spray 0.5kg of dry ice as a coolant during the superfine pulverization process ; At least 90% of the micropowder particle size in the superfine pulverization is not more than 5 microns; superfine pulverization to obtain ultrafine powder for later use; put polylactic acid and polyethylene glycol into the reaction kettle, and heat at 100 ° C, while heating Stir for 3 hours, then add phenytoin sodium superfine powder and continue to stir for 0.5 hours. When the auxiliary materials in the reaction kettle are in a viscous state, add methylcellulose sodium and continue to stir for 1 hour, and finally ad...
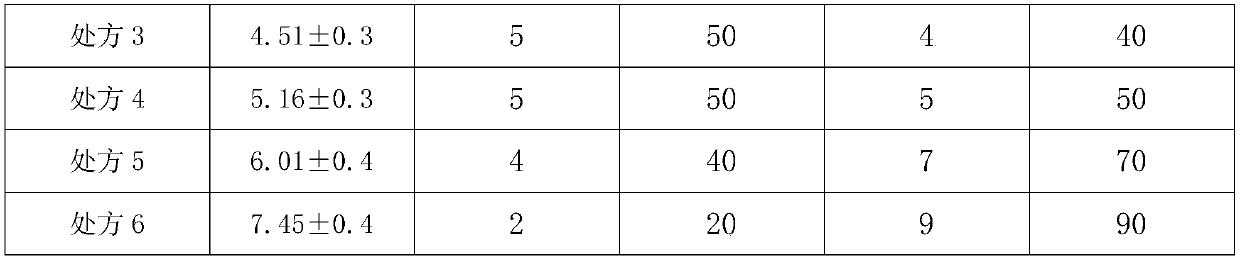
Embodiment 3
[0134] API:
[0135] Phenytoin 3.4kg, carbamazepine 0.9kg, lamotrigine 0.8kg;
[0136] Accessories:
[0137] Sodium methylcellulose 0.3kg, polylactic acid 0.25kg, polyethylene glycol 0.15kg.
[0138] Frozen phenytoin, carbamazepine and lamotrigine respectively at -15°C for 2 hours and then ultrafinely pulverized with a superfine pulverizer to obtain superfine powder; spray 0.5kg of dry ice as a coolant during the superfine pulverization process ; At least 90% of the micropowder particle size in the superfine pulverization is not more than 5 microns; superfine pulverization to obtain ultrafine powder for later use; put polylactic acid and polyethylene glycol into the reaction kettle, and heat at 100 ° C, while heating Stir for 3 hours, then add phenytoin sodium superfine powder and continue to stir for 0.5 hours. When the auxiliary materials in the reaction kettle are in a viscous state, add methylcellulose sodium and continue to stir for 1 hour, and finally add carbamazepine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com