Preparation method of carrier-free macrolide immunosuppressive drug nanoparticles
A technology of macrolides and immunosuppression, applied in drug combinations, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problem of uncertain carrier biological toxicity, cumbersome carrier preparation process, drug loading and encapsulation efficiency Low-level problems, to achieve the effect of solving potential toxicity, high drug loading efficiency, and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of carrier-free rapamycin self-assembled nanoparticles
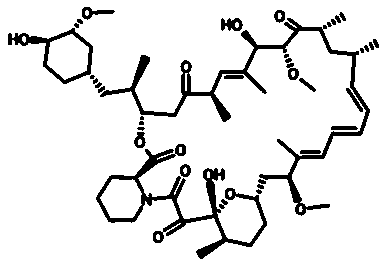
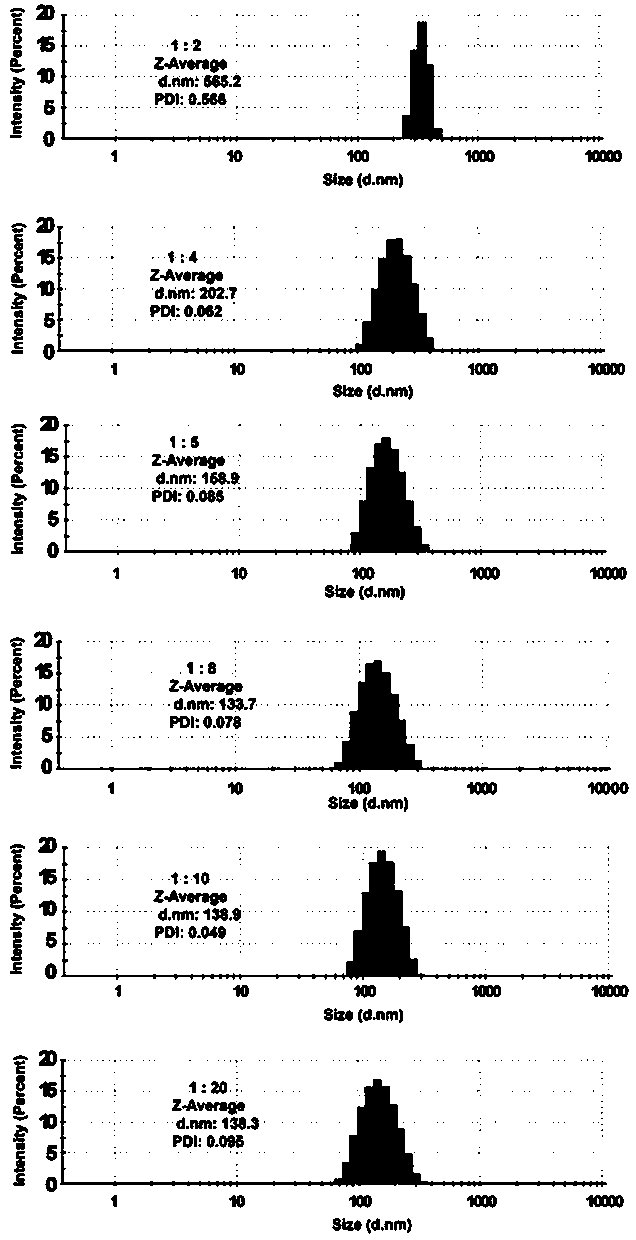
[0032] Accurately weigh 0.004 g of rapamycin powder, 6 parts in total, dissolve in 50 μL, 100 μL, 125 μL, 200 μL, 250 μL and 500 μL of methanol, dissolve by ultrasonication for 10 min, add dropwise to vortex shake In 1000μL of water, shake continuously for 0.5h~1h. Dry the organic solvent with pure nitrogen, sonicate for 30-60 min, centrifuge at 3000 rpm, and remove free drug for 10 min to obtain organic solvent-water ratios of 1:20, 1:10, 1:8, 1:5, and 1, respectively. :4 and 1:2 carrier-free rapamycin self-assembled nanoparticle solutions. The molecular structure of rapamycin is figure 1 As shown, the obtained particle size diagrams of different ratios of carrier-free rapamycin self-assembled nanoparticle solutions are shown in Figure 2.
[0033] When the ratio of organic solvent to water ranges from 1:2 to 1:20, the particle size gradually decreases to stabilize at around 130 nm, because the proc...
Embodiment 2
[0035] Determination of Drug Loading Capacity and Encapsulation Efficiency of Carrier-free Rapamycin Self-Assembled Nanoparticles
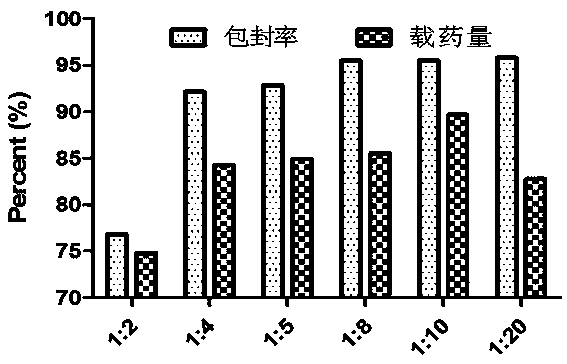
[0036] The carrier-free rapamycin self-assembled nanoparticle solutions of different proportions prepared in Example 1 were measured with a UV photometer, according to drug loading = (drug content in nanoparticles / total weight of nanoparticles) × 100 %, Encapsulation Efficiency = (Total Drug Amount - Free Drug Amount) / Total Drug Amount × 100 % Calculate the drug loading and encapsulation efficiency of each ratio. The result is as image 3 , each ratio has a higher drug loading and encapsulation efficiency, which is superior to the traditional organic or inorganic nano-drug loading system, and when the organic solvent-water ratio is 1:10, the particle size is moderate, the drug loading is 87.45% and The encapsulation rate is 95.53%, which is the best ratio.
Embodiment 3
[0038] Determination of the Morphology of Self-Assembled Nanoparticles of Carrier-Free Rapamycin
[0039] Carrier-free rapamycin self-assembled nanoparticles with an organic solvent-water ratio of 1:10 prepared according to the method of Example 1 were diluted 5 times with water, dropped on a flat and clean mica sheet surface for natural adsorption for 20 min, The surface of the mica flakes was then carefully cleaned with ultrapure water and immediately dried with nitrogen gas. The obtained samples were placed under the AFM instrument of the model Multimode 8 (Bruker, Germany) to observe the morphology through intelligent mode scanning imaging in air. The result is as Figure 4 As shown, the obtained carrier-free rapamycin self-assembled nanoparticles are round-shaped particles with relatively uniform size.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com