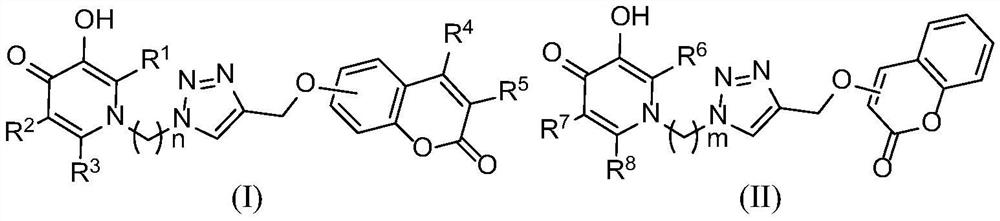
Coumarin/pyridone hybrid derivative and its preparation method and application
A technology of coumarin and derivatives, which is applied in drug combination, organic chemistry, nervous system diseases, etc., can solve the problems of low pertinence, complex pathogenesis of Alzheimer's disease, and single treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
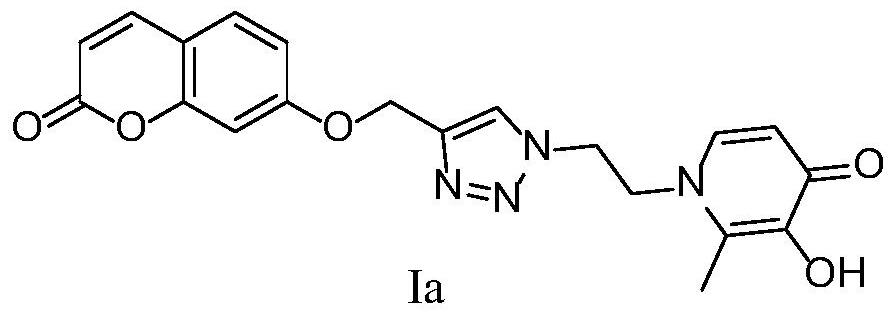
[0103] 2-Methyl-3-hydroxy-1-(2-(4-(((coumarin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl ) ethyl) the preparation method of pyridin-4-ketone (Ia)
[0104]
[0105] 7-(prop-2-yn-1-yloxy)-coumarin (1.2mmol), 1-(2-azidoethyl)-3-methoxy-2-methyl-4-one (1mmol), CuSO 4 ·5H 2 O (0.1 mmol), L-sodium ascorbate (0.5 mmol), MeOH (5 mL), H 2 O (5 mL), control the reaction temperature at 25°C, and react for 24h. The reaction was monitored by TLC. After the conversion of the raw materials was complete, the reaction was stopped, concentrated, and purified by silica gel column chromatography (dichloromethane:methanol=20:1 as eluent) to obtain a white solid intermediate.
[0106] Add the above-mentioned white intermediate (0.84mmol) and anhydrous dichloromethane (10mL) into a single-necked flask, and dissolve boron tribromide (3.6mmol) in anhydrous dichloromethane (10mL) and place in a constant pressure dropping funnel, N 2 Under protection, boron tribromide was slowly added dropwise at 0...
Embodiment 2
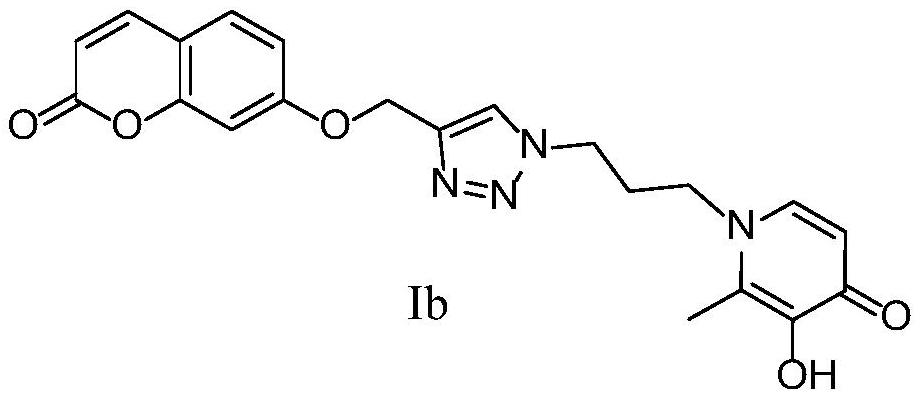
[0111] 2-Methyl-3-hydroxy-1-(3-(4-(((coumarin-7-yl)oxy)methyl)-1,2,3-triazol-1-yl)propane Base) the preparation method of pyridin-4-ketone (Ib)
[0112]
[0113] 7-(prop-2-yn-1-yloxy)-coumarin (1.2mmol), 1-(3-azidopropyl)-3-methoxy-2-methylpyridine-4- Ketone (1 mmol), CuSO 4 ·5H 2 O (0.1 mmol), L-sodium ascorbate (0.5 mmol), MeOH (5 mL), H 2 O (5 mL), control the reaction temperature at 25°C, and react for 24h. The reaction was monitored by TLC. After the conversion of the raw materials was complete, the reaction was stopped, concentrated, and purified by silica gel column chromatography (dichloromethane:methanol=20:1 as eluent) to obtain a white solid intermediate.
[0114] Add the above-mentioned white intermediate (0.82mmol) and anhydrous dichloromethane (10mL) into a single-necked flask, and dissolve boron tribromide (2.4mmol) in anhydrous dichloromethane (10mL) and place in a constant pressure dropping funnel, N 2 Under protection, boron tribromide was slowly add...
Embodiment 3
[0119] 2-(Hydroxymethyl)-3-hydroxy-6-methyl-1-(2-(4-(((coumarin-7-yl)oxy)methyl)-1,2,3-tri The preparation method of oxazol-1-yl) ethyl) pyridin-4-one (Ic)
[0120]
[0121] 7-(prop-2-yn-1-yloxy)-coumarin (1.2mmol), 1-(2-azidoethyl)-2-(hydroxymethyl)-3-methoxy- 6-Methylpyridin-4-one (1mmol), CuSO 4 ·5H 2 O (0.1 mmol), L-sodium ascorbate (0.5 mmol), MeOH (5 mL), H 2 O (5 mL), control the reaction temperature at 25°C, and react for 24h. The reaction was monitored by TLC. After the conversion of the raw materials was complete, the reaction was stopped, concentrated, and purified by silica gel column chromatography (dichloromethane:methanol=20:1 as eluent) to obtain a white solid intermediate.
[0122] Add the above-mentioned white intermediate (0.80mmol) and anhydrous dichloromethane (10mL) into a single-necked flask, and dissolve boron tribromide (2.4mmol) in anhydrous dichloromethane (10mL) and place in a constant pressure dropping funnel, N 2 Under protection, boron t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com