Synthetic method of epsilon-decalactone perfume
A synthesis method and technology of decanolide, applied in directions such as organic chemistry, can solve the problems of complicated operation, high cost, harsh reaction conditions, etc., achieve high reaction yield and reduce the effect of separation and purification procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
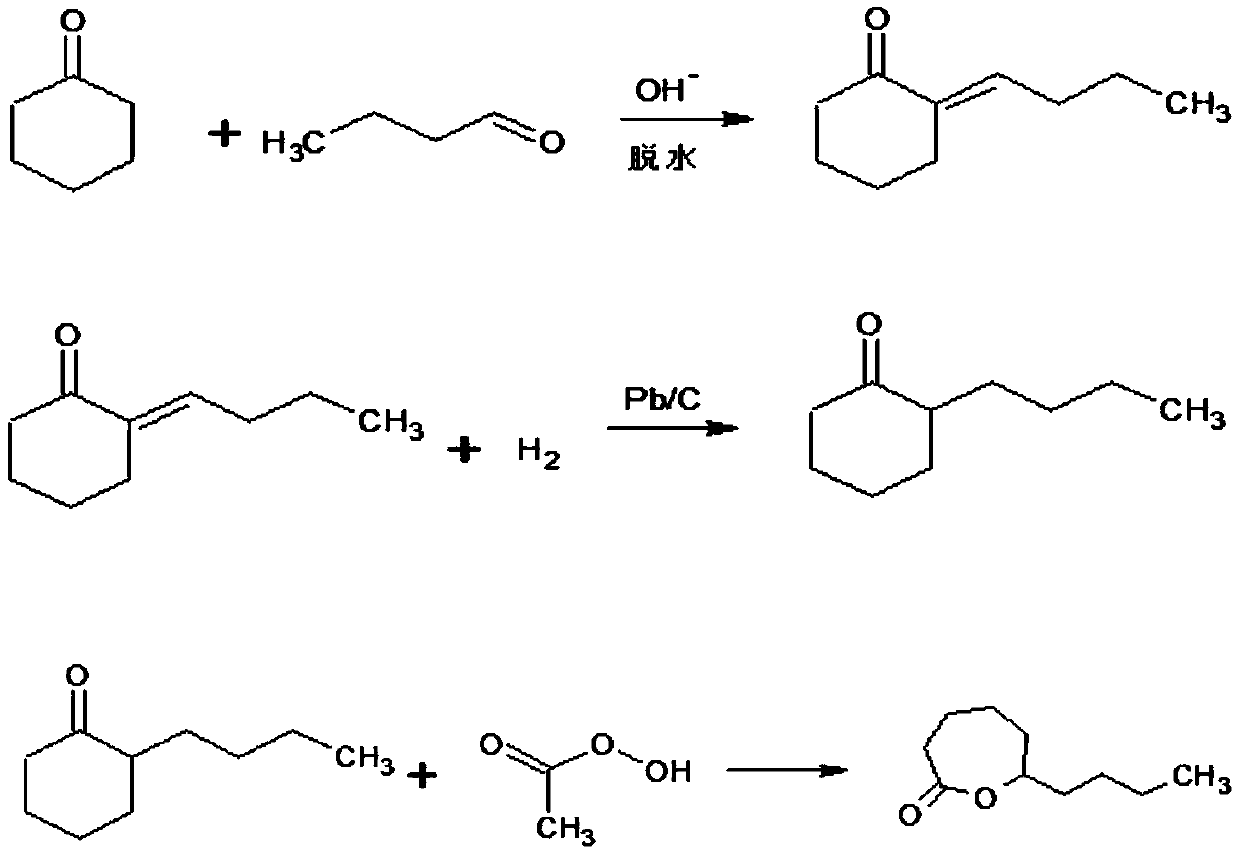
[0031] For this reason, the inventor of the present invention provides a kind of synthetic method of ε-decalactone perfume, comprises the following steps:
[0032] (1) Mix the alkaline solution and part of cyclohexanone uniformly under the condition of heating and stirring to obtain mixture A; then add dropwise the mixture B of n-butyraldehyde and the remaining amount of cyclohexanone to the mixture A, after the addition is completed, Continue to stir and insulate and react until the content of n-butyraldehyde in the reactant is lower than 1.0%;
[0033] (2) The reactant of step (1) is left to stand, and the water layer is collected for recycling; the oil layer is collected, and the oil layer is washed to neutrality, and then the layers are left to stand, and the oil layer is collected to obtain the condensation product 2-butenyl cyclohexanone Crude;
[0034] (3) Utilize the rotary evaporator to distill the 2-butenyl cyclohexanone crude product, reclaim the cyclohexanone and ...
Embodiment 1
[0063] A kind of synthetic method of ε-decalactone perfume:
[0064] S1: In a 1000mL three-necked flask equipped with a thermometer, a stirrer, and a peristaltic pump, add 500mL of sodium hydroxide solution with a mass fraction of 2.5%, turn on the agitator and a constant temperature water tank, then add 50g of cyclohexanone, and mix well to obtain a mixture A; heat up to 75°C, dropwise add a mixture B of 40g of n-butyraldehyde and 60g of cyclohexanone to a three-necked flask through a peristaltic pump to carry out aldol condensation reaction, stir while adding, dropwise is completed after 3h, and then continue to stir to make The above reactants were kept warm for 2 hours at 75°C, and the reaction was terminated when the n-butyraldehyde content in the reactants was detected to be lower than 1%.
[0065] S2: Lower the temperature, transfer the reactant to a separatory funnel and let it stand for stratification for 2 hours; collect the sodium hydroxide solution in the water lay...
Embodiment 2
[0075] A kind of synthetic method of ε-decalactone perfume:
[0076] S1: In a 500mL three-neck flask equipped with a thermometer, a stirrer, and a peristaltic pump, add 250mL of sodium hydroxide solution with a mass fraction of 2.5%, turn on the agitator and a constant temperature water tank, then add 30g of cyclohexanone, and mix well to obtain a mixture A; heat up to 80°C, drop a mixture B of 15g of n-butyraldehyde and 30g of cyclohexanone into a three-necked flask through a peristaltic pump to carry out aldol condensation reaction, adopt drop-adding and stirring technology, stir while dropping, and finish adding in 2 hours , and then continue to stir, so that the above reactants are kept warm for 2 hours at 80° C., and the reaction is terminated when the n-butyraldehyde content in the reactants is lower than 1%;
[0077] S2: Cool down to normal temperature, transfer the reactant to the separatory funnel and let it stand for stratification for 2 hours; collect the sodium hyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com