Preparation of Sorafenib Nanomedicine Based on Small Molecular Chaperones
A sorafenib nanometer and nanomedicine technology, which is applied in the field of preparation of sorafenib nanometer medicine, can solve the problems of high production cost, complicated preparation process and poor reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of Sorafenib Nanomedicine Based on Small Molecular Chaperone 6
[0049]
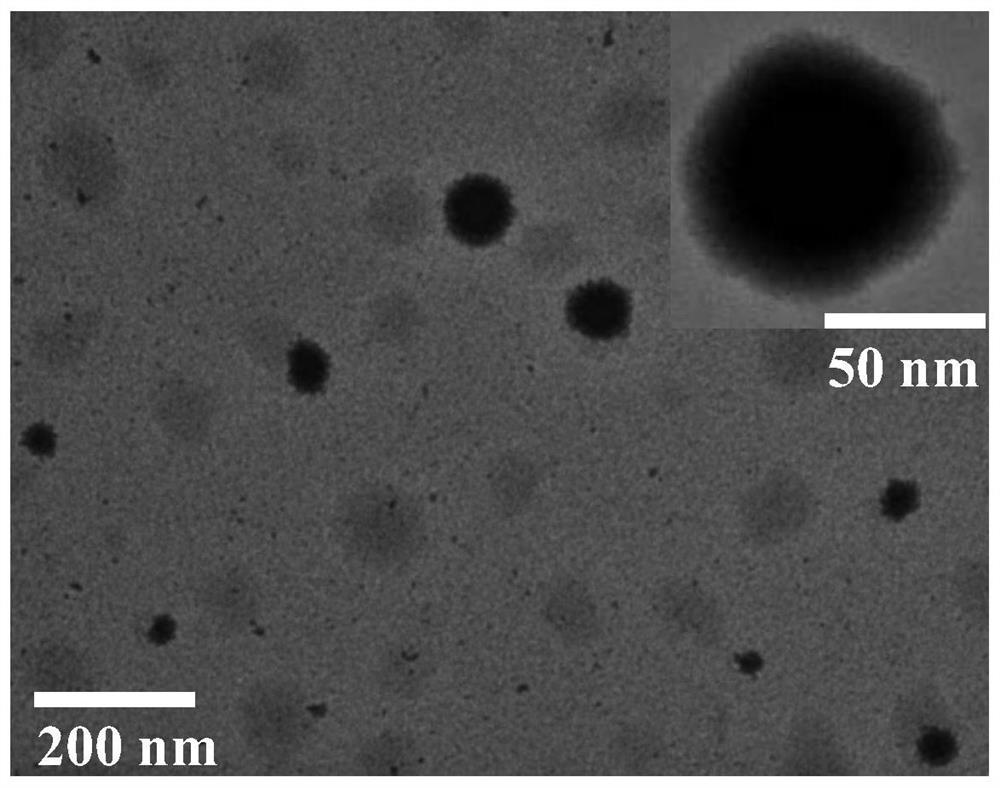
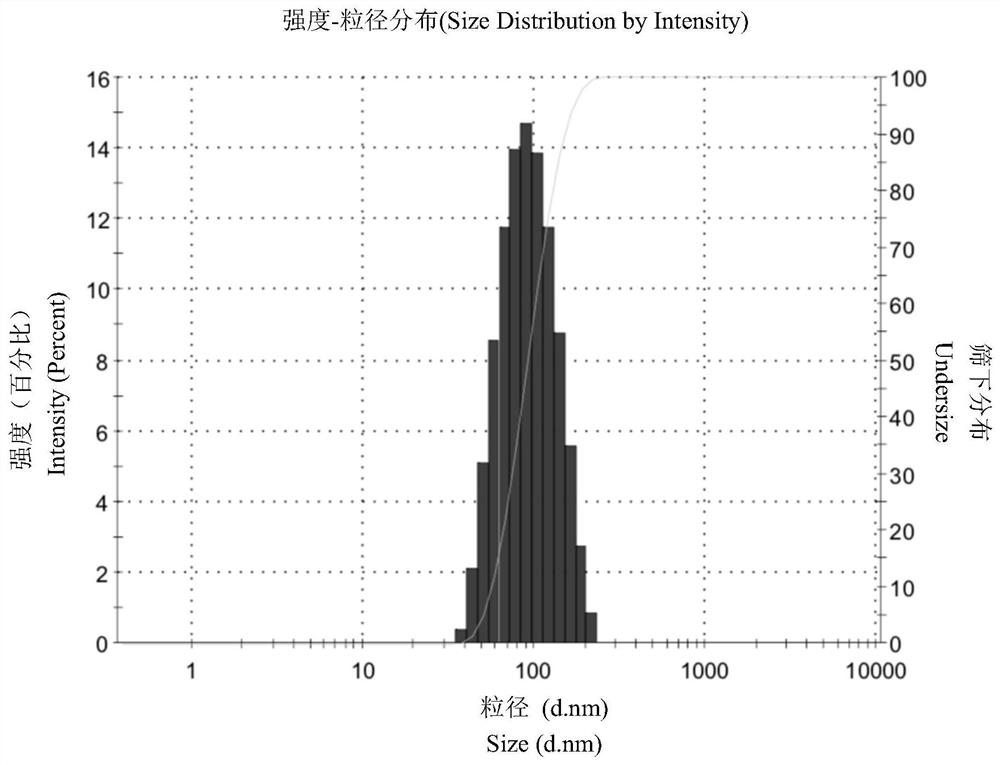
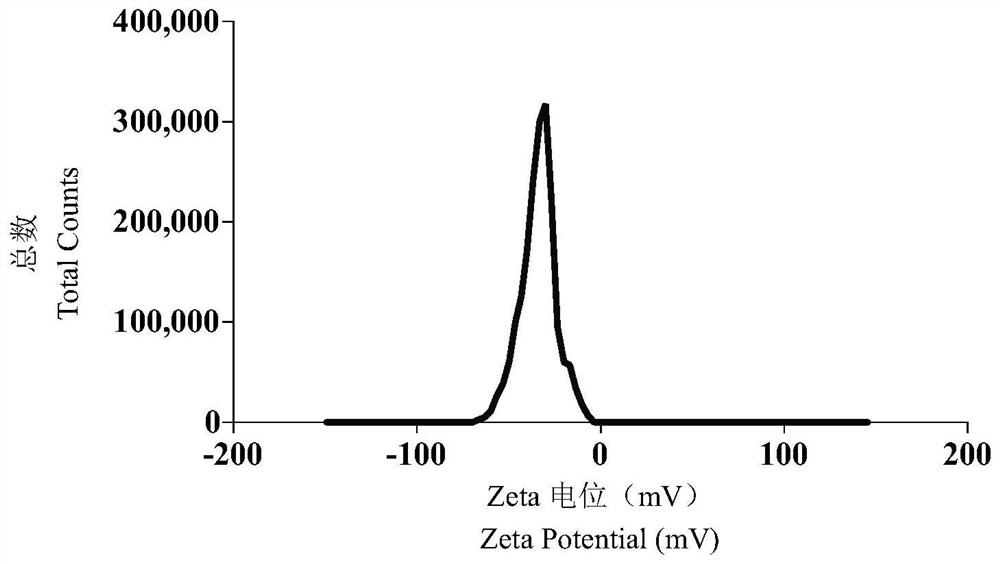
[0050] Dissolve sorafenib and small molecule chaperone 6 (molar ratio 1:1) in DMF (sorafenib 25mg / ml), and add 40ul DMF solution to 1000ul water for injection at room temperature with a dropping rate of 1mL / min, vortex stirring at high speed (2000r / min), and remove the organic solvent after dialysis to obtain sorafenib nanomedicine. Analysis and determination by transmission electron microscopy (TEM) and dynamic light scattering particle size analyzer (DLS). It is found that the nanoparticles formed by sorafenib under the action of 6 are 80-100nm, with round shape and good dispersion. figure 1 As shown, the dispersion coefficient PDI is 0.1~0.2, and the potential is -30~-40mV, such as figure 2 Shown.
Embodiment 2-7
[0052] According to the method in Example 1 and the same conditions as in Example 1, sorafenib nanomedicines with different small molecular chaperones were prepared respectively, and the results are shown in Table 1.
[0053] Table 1
[0054]
[0055]
Embodiment 8-11
[0057] Taking small molecular chaperone 6 as an example, in this example, a sorafenib nanomedicine based on small molecular chaperone 6 was prepared according to the method of Example 1, and different molar ratios of sorafenib and small molecular chaperone were prepared. The effects of sorafenib nano-drugs are shown in Table 2.
[0058] Table 2
[0059]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap