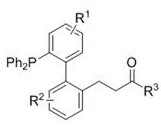
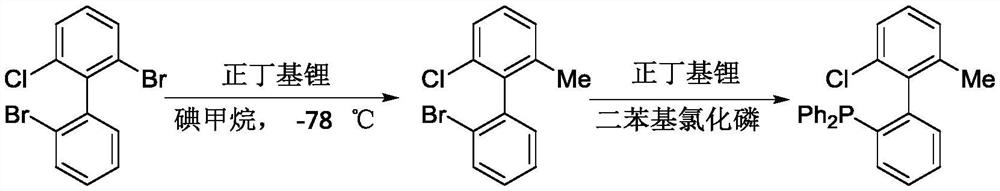
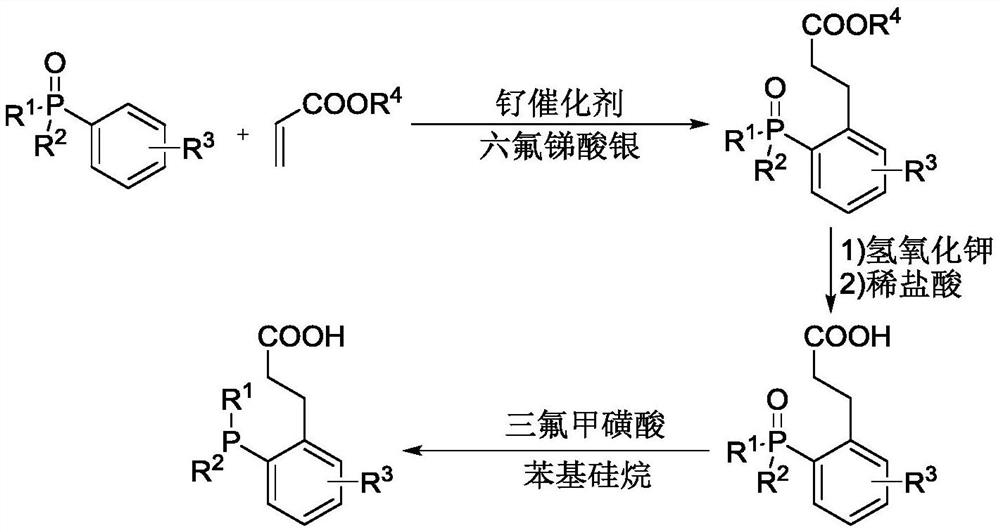
A Novel Alkylated Monophosphine Ligand and Its Simple Preparation Method
A technology of alkylation and ligands, applied in the field of new monophosphine ligands and their synthesis, new alkylated monophosphine ligands and their simple preparation, can solve the problem of single type of alkylation, complicated preparation process, and difficult operation process Complexity and other issues, to achieve the effect of reducing production costs, simple synthesis steps, and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The novel alkylated monophosphine ligand III-1{3-(2'-(diphenylphosphino)-[1,1'-biphenyl]-2-yl) ethyl propionate} of this example, The synthetic route is as follows:
[0036]
[0037] The above-mentioned novel alkylated monophosphine ligand III-1 is prepared by the following method, including the following steps:
[0038] Add 101.4 mg (0.3 mmol) 2-diphenylphosphine-biphenyl (compound I-1), 82.5 μL ethyl acrylate (compound II-1, 0.76 mmol), 9.3 mg p-cymene dichloride to the reaction kettle Ruthenium dimer, 15.8mg N-Boc-glycine, 5.3mg tris(4-methoxyphenyl) phosphine, 58.8mg potassium acetate and 1.5mL n-hexane, stirred and mixed evenly, then passed argon into the reaction system, The reaction was controlled in an argon atmosphere at 120° C. for 24 hours, then cooled to room temperature, filtered with diatomaceous earth, vacuum distilled, and separated by column chromatography to obtain 93.8 mg of the target product with a yield of 71%.
[0039] The target product test r...
Embodiment 2
[0048] The new alkylated monophosphine ligand III-2{3-(2'-(diphenylphosphino)-[1,1'-biphenyl]-2-yl) tert-butyl propanoate }, the synthetic route is as follows:
[0049]
[0050] The above-mentioned novel alkylated monophosphine ligand III-2 is prepared by the following method, including the following steps:
[0051] Add 101.4 mg (0.3 mmol) 2-diphenylphosphine-biphenyl (compound I-2), 141 μL tert-butyl acrylate (compound II-2), 9.3 mg p-cymene dichloride ruthenium dichloride to the reaction kettle Polymer, 15.8mg N-Boc-glycine, 5.3mg tris(4-methoxyphenyl) phosphine, 58.8mg potassium acetate and 1.5mL n-hexane, stirred and mixed evenly, and passed argon gas into the reaction system to control the reaction at After reacting at 120° C. for 24 h in an argon atmosphere, it was cooled to room temperature, filtered with diatomaceous earth, vacuum distilled, and separated by column chromatography to obtain 101 mg of the target product with a yield of 72%.
[0052] The target produc...
Embodiment 3
[0058] The novel alkylated monophosphine ligand III-3{3-(2'-(diphenylphosphoryl)-[1,2'-methoxybiphenyl]-2-yl)propane of this example Acid ethyl ester}, the synthetic route is as follows:
[0059]
[0060] The above-mentioned novel alkylated monophosphine ligand III-3 is prepared by the following method, including the following steps:
[0061] Add 110.9 mg (0.3 mmol) 2-methoxy-2-diphenylphosphine-biphenyl (compound I-3), 82.5 μL ethyl acrylate, 9.3 mg p-cymene dichloride ruthenium dichloride to the reaction kettle Polymer, 15.8mg N-Boc-glycine, 5.3mg tris(4-methoxyphenyl) phosphine, 58.8mg potassium acetate and 1.5mL n-hexane, stirred and mixed evenly, and passed argon gas into the reaction system to control the reaction at The reaction was carried out at 160° C. for 24 h in an argon atmosphere, cooled to room temperature, filtered with diatomaceous earth, vacuum distilled, and separated by column chromatography to obtain 115 mg of the target product with a yield of 71%.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com