Solid dispersion system containing lurasidone hydrochloride and preparation thereof
A technology of lurasidone hydrochloride and solid dispersion, applied in the field of pharmaceutical preparations, can solve the problems of affecting bioavailability, incomplete dissolution, slow dissolution rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Lurasidone Hydrochloride-Polyethylene Glycol 6000 Solid Dispersion and Preparation
[0019] 1. Preparation of lurasidone hydrochloride-polyethylene glycol 6000 solid dispersion
[0020] Lurasidone Hydrochloride 25g Macrogol 6000 50g Methanol 100g
[0021] Add lurasidone hydrochloride to the molten polyethylene glycol 6000, control the temperature at 40-50°C, add methanol dropwise while stirring until the solid is completely dissolved, keep stirring for 15 minutes, distill methanol under reduced pressure at 50-60°C, and the solid in Solidify below -15°C for 2 hours, pulverize, and pass through a 100-mesh sieve to obtain lurasidone hydrochloride solid dispersion.
[0022] 2. Preparation of preparations
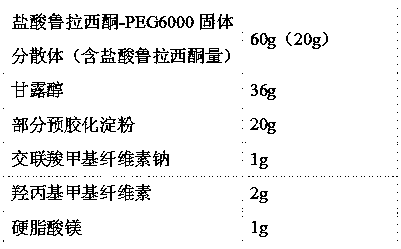
[0023] The lurasidone hydrochloride solid dispersion prepared above is mixed in proportion with the following pharmaceutical excipients to prepare tablets:
[0024]
[0025] Preparation Process:
[0026] 1) Lurasidone hydrochloride-polyethylene glycol 60...
Embodiment 2
[0030] Example 2: Lurasidone Hydrochloride-Polyethylene Glycol 4000 Solid Dispersion and Preparation
[0031] 1. Preparation of lurasidone hydrochloride-polyethylene glycol 4000 solid dispersion
[0032] Lurasidone Hydrochloride 25g Macrogol 4000 50g Methanol 50g
[0033] Add lurasidone hydrochloride to the molten polyethylene glycol 4000, control the temperature at 40-50°C, add methanol dropwise while stirring until the solid is completely dissolved, keep stirring for 15 minutes, distill methanol under reduced pressure at 50-60°C, and the solid in Solidify below -15°C for 2 hours, pulverize, and pass through a 100-mesh sieve to obtain lurasidone hydrochloride solid dispersion.
[0034] 2. Preparation of preparations
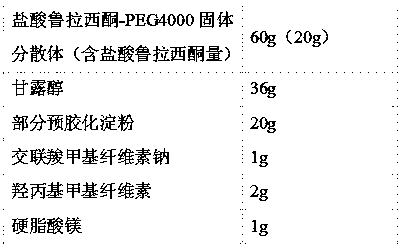
[0035] The lurasidone hydrochloride solid dispersion prepared above is mixed in proportion with the following pharmaceutical excipients to prepare tablets:
[0036]
[0037] Preparation Process:
[0038] 1) Lurasidone hydrochloride-polyethylene glycol 400...
Embodiment 3
[0042] Example 3: Lurasidone Hydrochloride-Poloxamer 188 Solid Dispersion and Preparation
[0043] 1. Preparation of lurasidone hydrochloride-poloxamer 188 solid dispersion
[0044] Lurasidone Hydrochloride 50g Poloxamer 188 100g Methanol 150g
[0045]Add lurasidone hydrochloride to the molten poloxamer 188, control the temperature at 40-50°C, add methanol dropwise while stirring until the solid is completely dissolved, keep stirring for 15 minutes, distill the methanol under reduced pressure at 50-60°C, and the solid in Solidify below -15°C for 2 hours, pulverize, and pass through a 100-mesh sieve to obtain lurasidone hydrochloride solid dispersion.
[0046] 2. Preparation of preparations
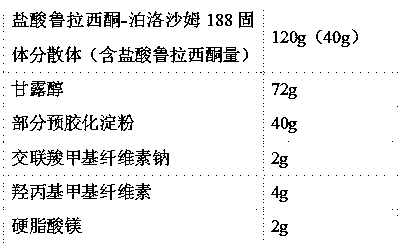
[0047] The lurasidone hydrochloride solid dispersion prepared above is mixed in proportion with the following pharmaceutical excipients to prepare tablets:
[0048]
[0049] Preparation Process:
[0050] 1) Lurasidone hydrochloride-poloxamer 188 solid dispersion, mannitol, partially...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com