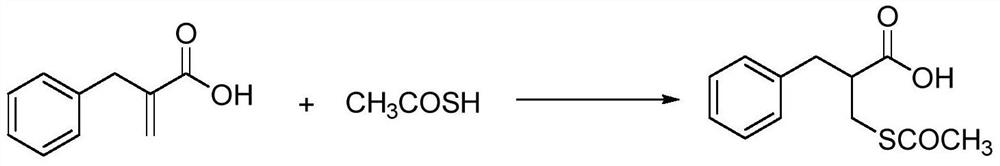A kind of process for preparing cadoxtril by 3-phenyl-1-propyne
A process method, the technology of Cardotril, which is applied in the field of pharmaceutical intermediates, can solve the problems of complex production process, increased work difficulty, and many reaction steps, and achieve the effects of low cost, energy saving, and simplified production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
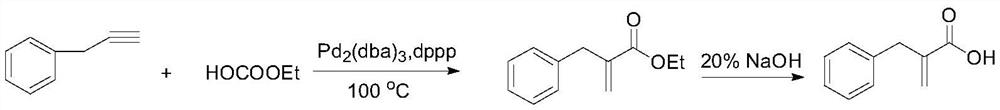
[0014] Example 1: Benzyl Acrylic Acid
[0015]
[0016] In a 500mL three-necked flask, 3-phenyl-1-propyne (11.6g, 0.1moL), monoethyl carbonate (9g, 0.1moL), Pd 2 (dba) 3 (0.46g, 0.01moL) , dppp (0.4g, 0.02moL), and finally add 200mL solvent anhydrous toluene, stir for 10min under nitrogen protection, and slowly raise the temperature to 100°C. React for 24 hours. After the reaction, the reaction liquid was cooled to room temperature. Filter the catalyst, then add 100mL of 20% NaOH solution, heat to reflux for 2h, cool to room temperature and let stand to separate layers, add concentrated hydrochloric acid to adjust the pH=1, extract the aqueous phase with ethyl acetate, combine the organic phase, spin to dry the solvent, and dry in vacuum; Recrystallization with ethanol gave 13 g of white solid benzyl acrylic acid. Yield 80%. MS(EI):m / z:162.07([M] + ).
Embodiment 2
[0017] Example 2: 2-acetylthiomethyl-3-phenylpropionic acid
[0018]
[0019] Add benzylacrylic acid (13g, 0.08moL) and thioacetic acid (7.3g, 0.096moL) into a 500mL three-necked reaction flask, and stir at 100°C for 2h; Thioacetic acid, then add 100mL toluene solvent, continue pressure distillation to completely remove thioacetic acid, recrystallize with ethanol to obtain white solid 2-acetylthiomethyl-3-phenylpropionic acid 17g. Yield 90%. MS(EI):m / z:238.07([M] + ).
Embodiment 3
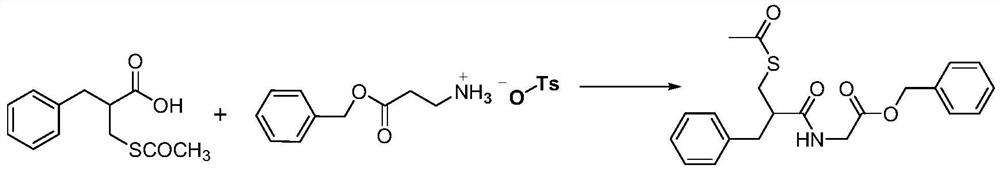
[0020] Embodiment 3: Cardotril
[0021]
[0022] Add 2-acetylthiomethyl-3-phenylpropionic acid (17g, 0.072moL) and solvent 150mL DMF into a 500mL three-necked reaction flask, cool to 0°C, add glycine benzyl ester p-toluenesulfonate (24.2 g, 0.072moL), triethylamine 10mol, and finally add HOBT (11g, 0.072moL), DCC (15.7g, 0.072moL) DMF solution 30mL. Stir for 1 h, then rise to room temperature and stir for 15 h. After the reaction is completed, filter out the DMF solvent to obtain the crude product cadotril, which is recrystallized with ethanol to obtain 19.4 g of white crystalline powder with a yield of 70%. MS(EI):m / z:385.13([M] + ).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap