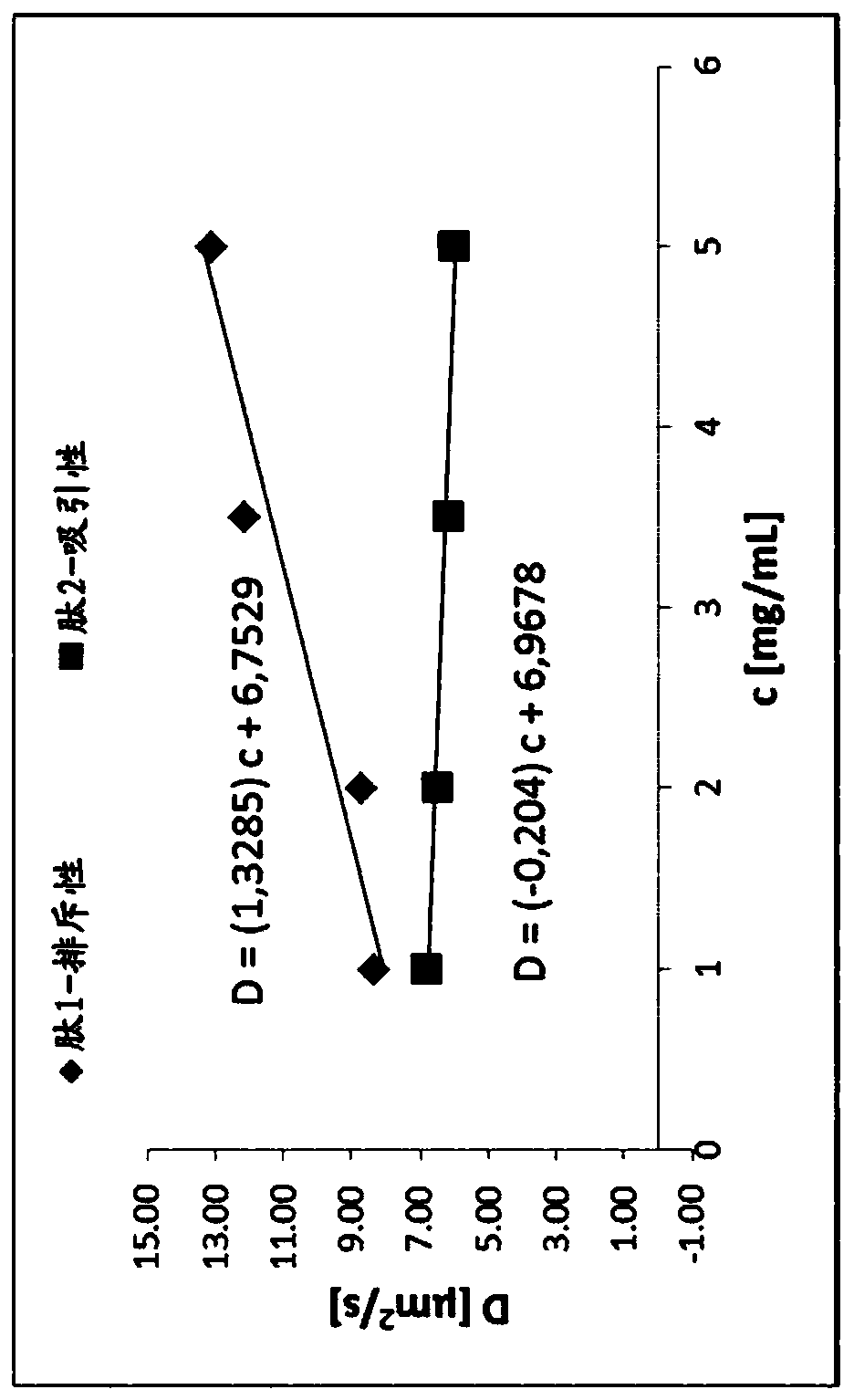
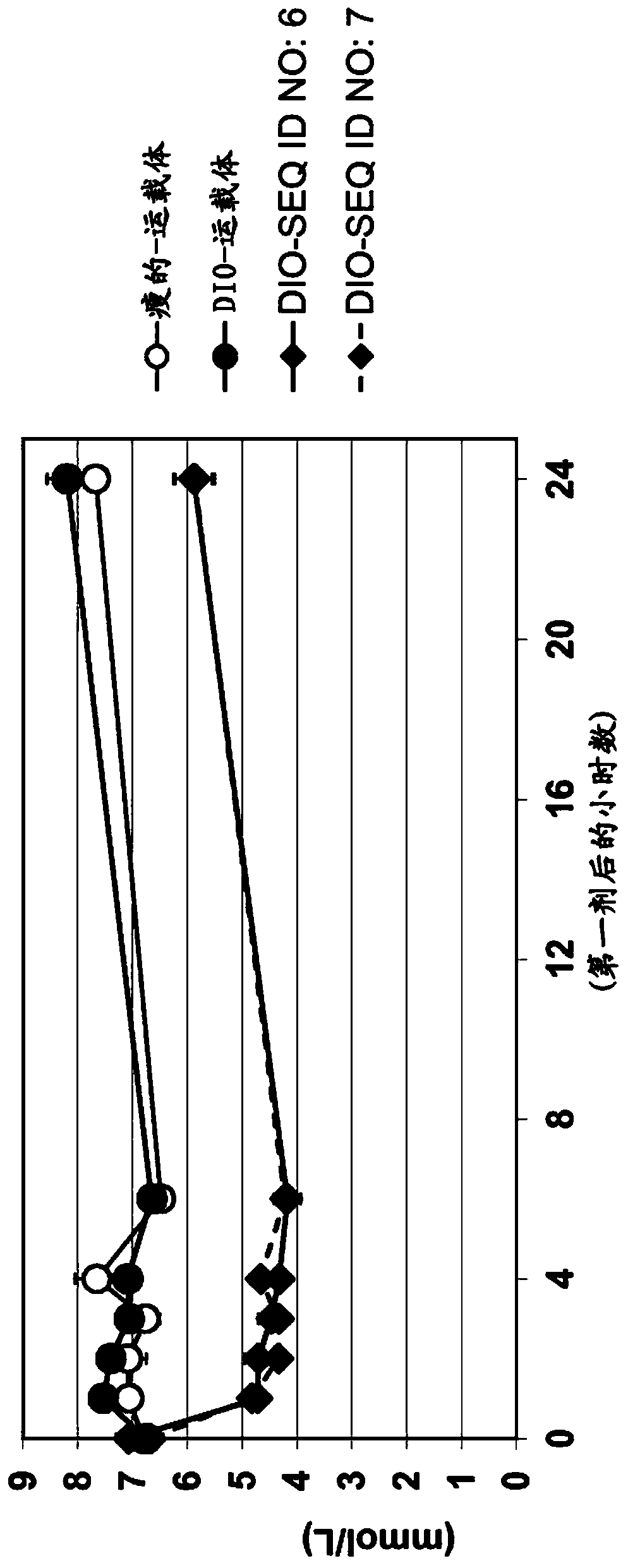
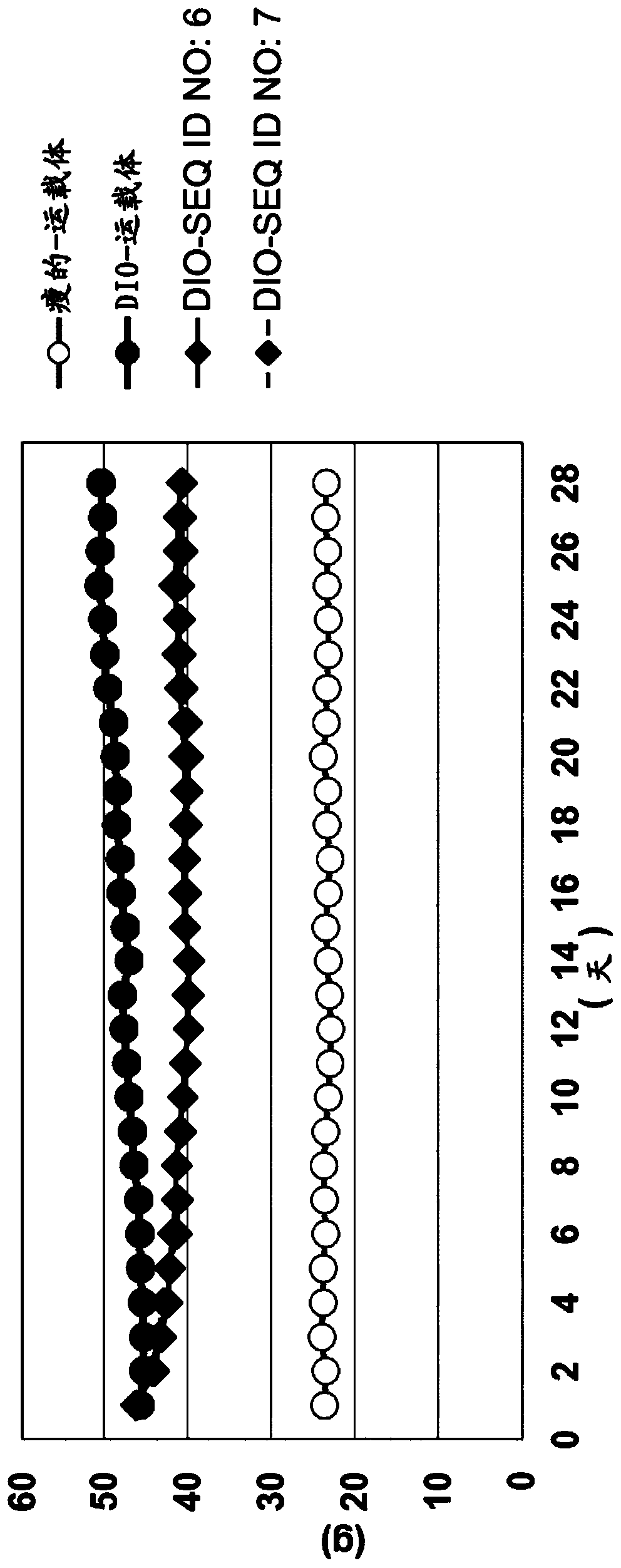
New compounds as peptidic GLP1/glucagon/GIP receptor agonists
A compound and solvate technology, applied in the field of new compounds as peptide GLP1/glucagon/GIP receptor agonists, can solve the problems of chemical instability of Exendin-4
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0426] Synthesis of SEQ ID NO: 6
[0427] In Novabiochem Rink-amide resin (4-(2',4'-dimethoxypentyl-Fmoc-aminomethyl)-phenoxyacetamido-n-leucylaminomethyl resin), 100-200 mesh The solid-phase synthesis as described in the method was performed on a loading of 0.43mmol / g. The Fmoc synthesis strategy is applied together with HBTU / DIPEA activation. Fmoc-Lys(Mmt)-OH in position 14 and Boc-His(Trt)-OH in position 1 were used in the solid phase synthesis scheme. Cut the Mmt group from the peptide on the resin as described in the method. Thereafter, DIPEA was used as the base to couple Palm-gGlu-gGlu-OSu to the released amino group. The peptide was cleaved from the resin with King's cocktail mix (D.S. King, C.G. Fields, G.B. Fields, Int. J. Peptide Protein Res. 1990, 36, 255-266). The crude product was purified via preparative HPLC on a Waters column (SunfirePrep C18 ODB 5μm 50x150mm) using an acetonitrile / water gradient (both buffers containing 0.1% TFA). The purified peptide was a...
Embodiment 2
[0430] Synthesis of SEQ ID NO: 7
[0431] In Novabiochem Rink-amide resin (4-(2',4'-dimethoxypentyl-Fmoc-aminomethyl)-phenoxyacetamido-n-leucylaminomethyl resin), 100-200 mesh The solid-phase synthesis as described in the method was performed on a loading of 0.43mmol / g. The Fmoc synthesis strategy is applied together with HBTU / DIPEA activation. Fmoc-Lys(Mmt)-OH in position 14 and Boc-His(Trt)-OH in position 1 were used in the solid phase synthesis scheme. Cut the Mmt group from the peptide on the resin as described in the method. Thereafter, DIPEA was used as the base to couple Palm-gGlu-gGlu-OSu to the released amino group. The peptide was cleaved from the resin with King's cocktail mix (D.S. King, C.G. Fields, G.B. Fields, Int. J. Peptide Protein Res. 1990, 36, 255-266). The crude product was purified via preparative HPLC on a Waters column (SunfirePrep C18 ODB 5μm 50x150mm) using an acetonitrile / water gradient (both buffers containing 0.1% TFA). The purified peptide was a...
Embodiment 3
[0434] Synthesis of SEQ ID NO: 11
[0435] In Novabiochem Rink-amide resin (4-(2',4'-dimethoxypentyl-Fmoc-aminomethyl)-phenoxyacetamido-n-leucylaminomethyl resin), 100-200 mesh The solid-phase synthesis as described in the method was performed on a loading of 0.43mmol / g. The Fmoc synthesis strategy is applied together with HBTU / DIPEA activation. Fmoc-Lys(Mmt)-OH in position 14 and Boc-His(Trt)-OH in position 1 were used in the solid phase synthesis scheme. Cut the Mmt group from the peptide on the resin as described in the method. Thereafter, DIPEA was used as the base to couple Palm-gGlu-gGlu-OSu to the released amino group. The peptide was cleaved from the resin with King's cocktail mix (D.S. King, C.G. Fields, G.B. Fields, Int. J. Peptide Protein Res. 1990, 36, 255-266). The crude product was purified via preparative HPLC on a Waters column (SunfirePrep C18 ODB 5μm 50x150mm) using an acetonitrile / water gradient (both buffers containing 0.1% TFA). The purified peptide was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com