Pharmaceutical composition for prevention or treatment of cardiovascular diseases accompanied by diabetes, including amlodipine, losartan, and rosuvastatin, and composite preparation including the same
A technology of rosuvastatin and amlodipine, which is applied in the fields of cardiovascular system diseases, drug combinations, medical preparations containing active ingredients, etc., can solve the problems of undisclosed diabetes patients such as blood pressure and lipid concentration, and enhance the convenience of medication Sexuality and medication adherence, lower mean diastolic blood pressure, excellent blood pressure normalization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0131] Example 1: Selection of Trial Subjects and Administration of Clinical Trial Drugs
[0132] 1. Selection of test subjects
[0133] Trial subjects were selected based on the following selection criteria:
[0134] 1) Adults aged 19 to 75
[0135] 2) Patients who meet the following conditions during the first screening
[0136] ①Blood pressure standard: sitDBP≥90 mm Hg
[0137] ②Lipid standard: LDL-C≤250 mg / dl, TG<400 mg / dl
[0138] 3) Patients who meet the following conditions at the second visit after 4 weeks of therapeutic lifestyle change (TLC)
[0139] ① Blood pressure standard: 80 mm Hg ≤ sitDBP < 110 mm Hg
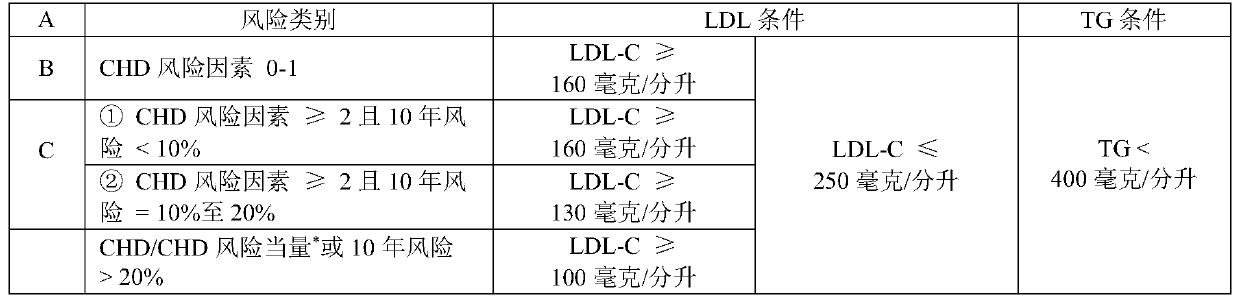
[0140] ②Lipid criteria: Patients who meet the following conditions when classified into Group A, Group B, and Group C according to cardiovascular disease risk
[0141]
[0142] Coronary Heart Disease (CHD) Risk Factors
[0143] 1) Current smoker
[0144] 2) Taking antihypertensive drugs
[0145] 3) Low HDL-C (
[0146] 4) Early family history of ...
experiment example 1
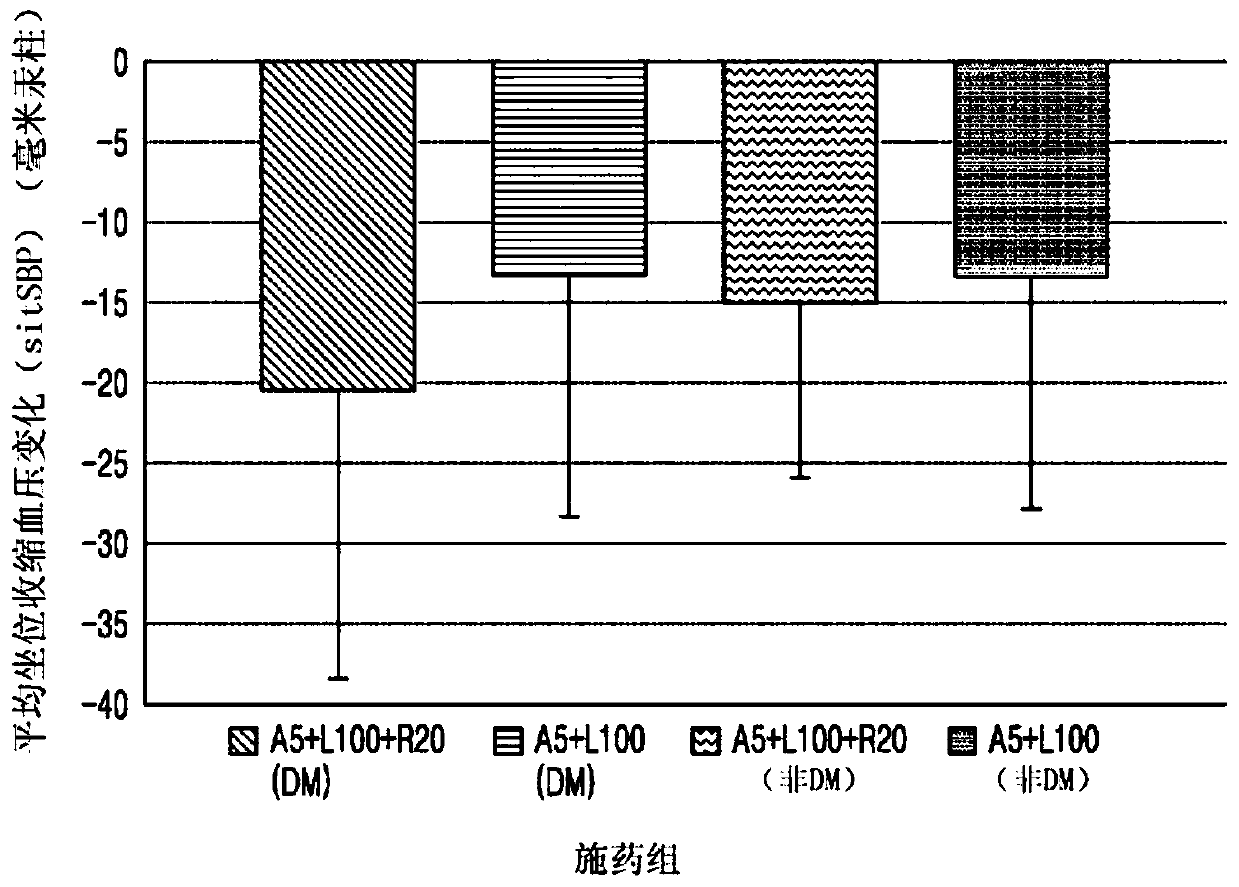
[0209] Experimental Example 1: Measuring mean sitting systolic blood pressure in a diabetic (DM) patient group compared to a non-diabetic patient group (sitSBP) reduction (mmHg)
[0210] For the group (A5+L100+R20) administered with 5 mg of amlodipine, 100 mg of rosuvastatin and 20 mg of rosuvastatin (experimental group) and the group administered with 5 mg of amlodipine and 100 mg of rosuvastatin (A5+L100 ) (control group 1), the change in mean sitSBP relative to baseline (mmHg) of both was measured after 8 weeks. The experimental group and the control group each included a diabetic patient group and a non-diabetic patient group. The results are shown in Table 1 and figure 1 shown.
[0211] [Table 1]
[0212] a): A5+L100+R20(DM) to A5+L100(DM)
[0213]
[0214] b): A5+L100+R20 (DM) to A5+L100 (non-DM)
[0215] c): A5+L100+R20 (non-DM) to A5+L100 (non-DM)
[0216] as table 1 and figure 1 As shown, the (A5+L100+R20) administration group exhibited a larger change i...
experiment example 2
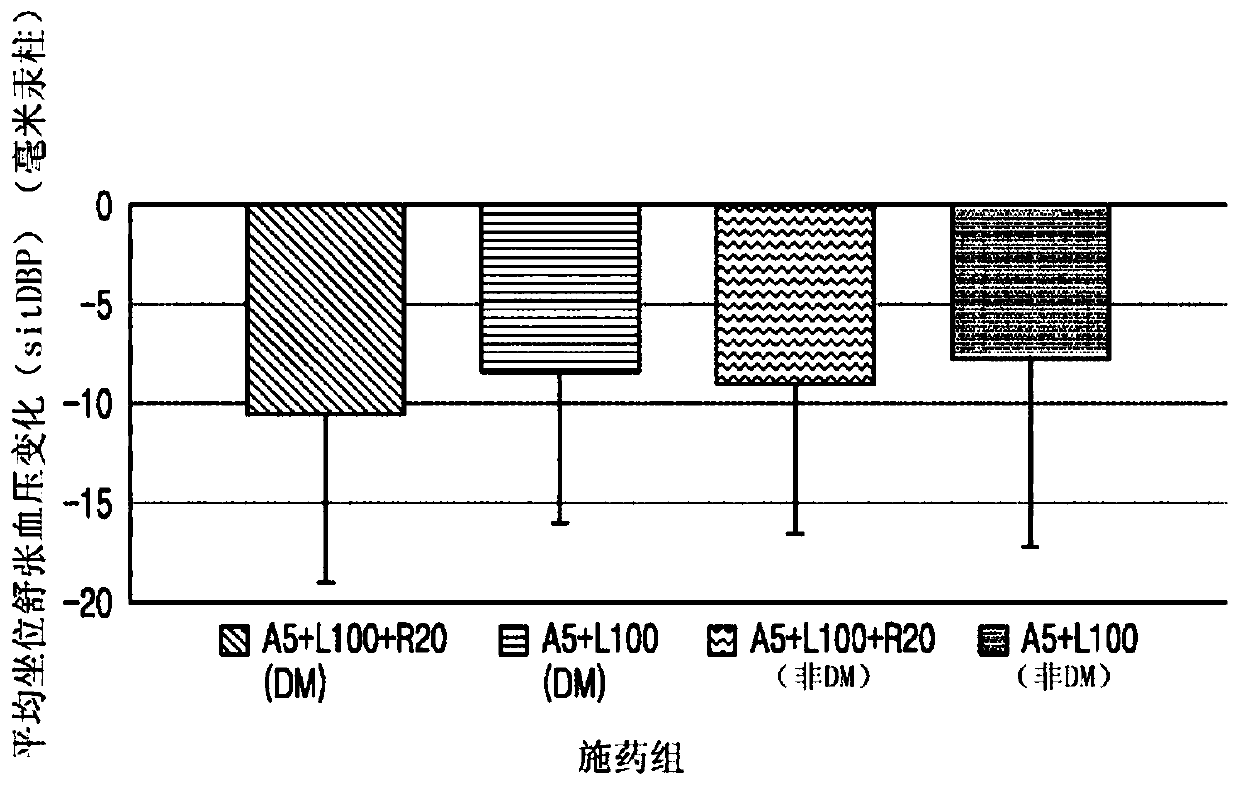
[0219] Experimental Example 2: Measuring the mean sitting diastolic blood pressure of the diabetic group compared to the non-diabetic group (sitDBP) reduction (mmHg)
[0220] The change from baseline in mean sitDBP (mmHg) was measured after 8 weeks in the group (A5+L100+R20) (experimental group) administered 5 mg amlodipine, 100 mg rosuvastatin, and 20 mg rosuvastatin . The experimental group and the control group each include a diabetic patient group and a non-diabetic patient group. The results are shown in Table 2 and figure 2 shown.
[0221] [Table 2]
[0222]
[0223] a): A5+L100+R20(DM) to A5+L100(DM)
[0224] b): A5+L100+R20 (DM) to A5+L100+R20 (non-DM)
[0225] c): A5+L100+R20 (non-DM) to A5+L100 (non-DM)
[0226] as table 2 and figure 2 As shown, the (A5+L100+R20) administration group exhibited an increase in sitDBP change (mm Hg) after 8 weeks compared to the (A5+L100) administration group. From this result it can be seen that when 5 mg amlodipine, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com