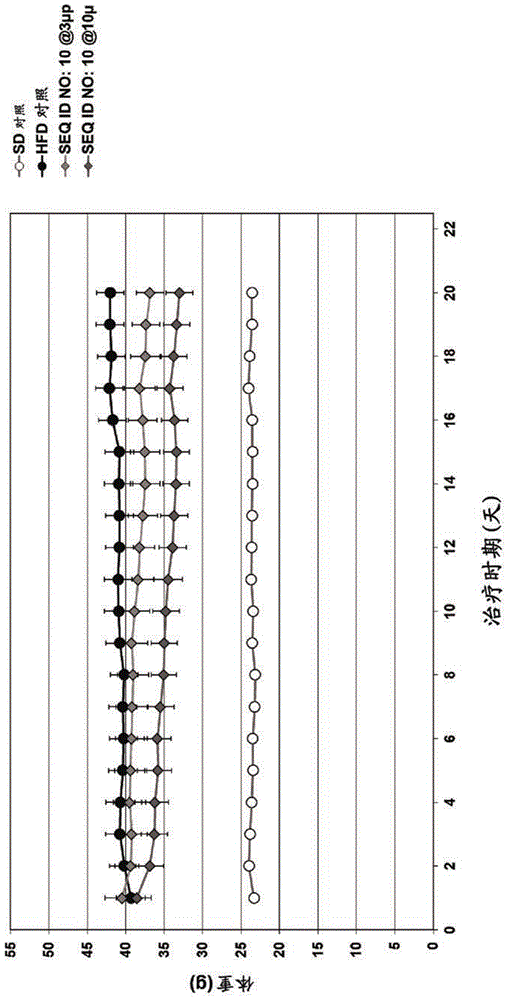
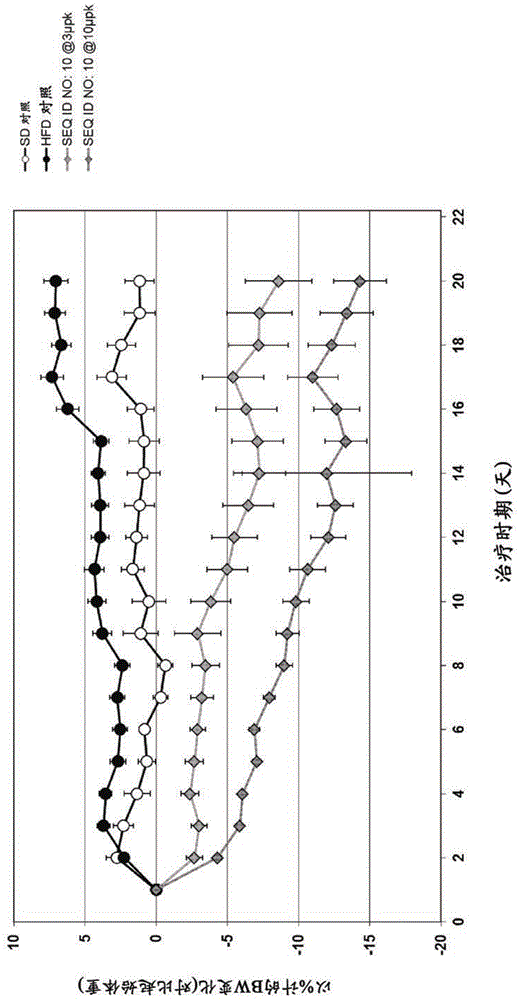
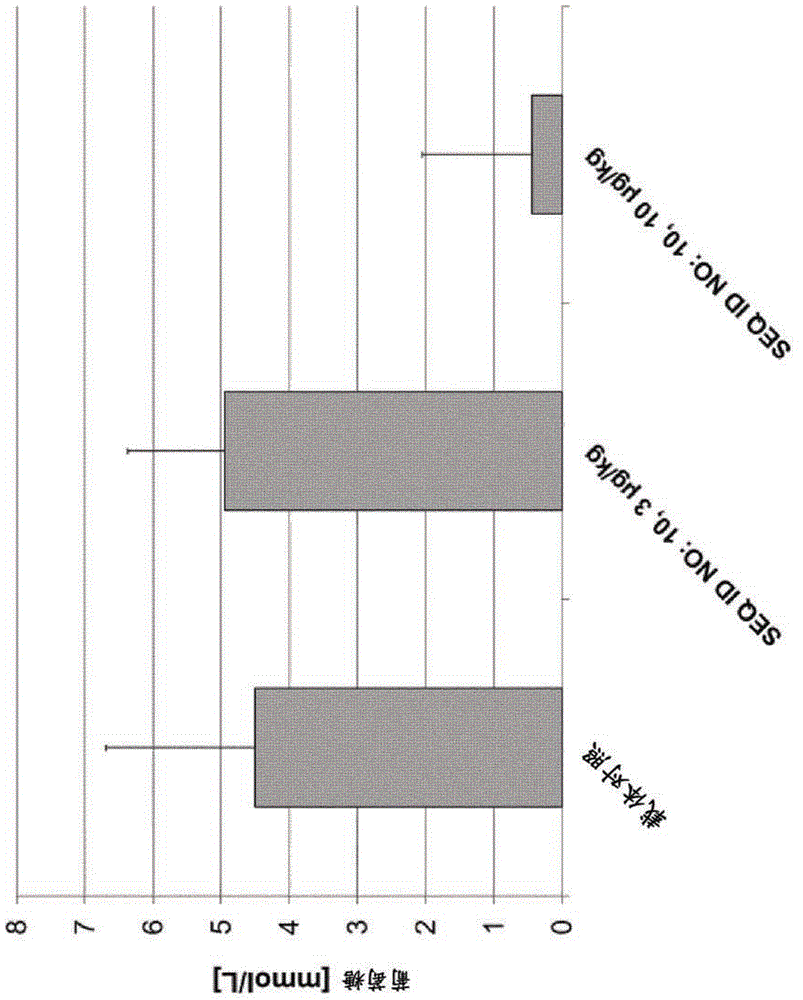
Exendin-4 derivatives
A peptide compound, compound technology, applied in the direction of antitoxins, hormone peptides, specific peptides, etc., can solve problems such as chemical instability of Exendin-4
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0352] definition
[0353] The amino acid sequences of the present invention contain the conventional one-letter or three-letter codes for naturally occurring amino acids, as well as the generally accepted three-letter codes for other amino acids, such as Aib (alpha-aminoisobutyric acid), Orn (ornithine ), Dab (2,4-diaminobutyric acid), Dap (2,3-diaminopropionic acid), Nle (norleucine), GABA (γ-aminobutyric acid) or Ahx (ε-aminocaproic acid )
[0354] In addition, the following codes were used for the amino acids shown in Table 4.
[0355] Table 4:
[0356]
[0357] The term "natural exendin-4" means a compound having the sequence HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPS-NH 2 Native Exendin-4 of (SEQ ID NO: 1).
[0358] The present invention provides a peptide compound as defined above.
[0359] The peptidic compounds of the present invention comprise a linear backbone of aminocarboxylic acids linked by peptide bonds, ie, carboxamide bonds. Preferably, the aminocarboxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com