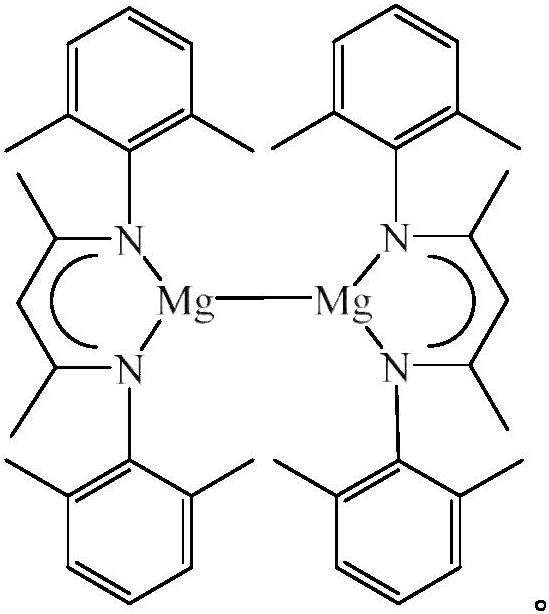
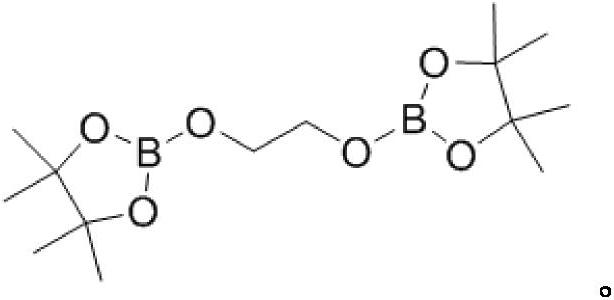
A kind of hydroboration method of organic carbonate
A carbonate and hydroboration technology, which is applied in the field of hydroboration reaction, achieves the effects of high catalytic efficiency, wide substrate universality and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The β-diimine monovalent magnesium compound catalyzes the reaction of ethylene carbonate and pinacol borane, and the process is as follows:
[0018] In the glove box, 1mol% of β-diimine monovalent magnesium compound, 0.4mmol of ethylene carbonate, and 1.6mmol of pinacol borane were successively added to the reaction bottle, and then it was removed from the glove box, stirred for 6h, and tested by nuclear magnetic The spectrum gave a 99% yield.
[0019] Characterize the product, the data are: 1 H NMR (600MHz, CDCl 3 ): 63.86 (s, 4H, OCH 2 ), 1.18(s, 24H, BOCMe 2 ). 13 C{ 1 H} NMR (151MHz, CDCl 3 ): δ82.65 (BOCMe 2 ), 64.98 (OCH 2 ), 24.53 (BOCMe 2 ). 11 B{ 1 H} NMR (193MHz, CDCl 3 ): δ22.24.
[0020] Available, the product structural formula is:
[0021]
Embodiment 2
[0023] The β-diimine monovalent magnesium compound catalyzes the reaction of propylene carbonate and pinacol borane, and the process is as follows:
[0024] In the glove box, 1mol% of β-diimine monovalent magnesium compound, 0.4mmol of propylene carbonate, and 1.6mmol of pinacol borane were successively added to the reaction bottle, and then it was removed from the glove box, stirred for 6h, and analyzed by nuclear magnetic spectrum. The figure gives a yield of 99%.
[0025] Characterize the product, the data are: 1 H NMR (600MHz, CDCl 3 ): δ4.23-4.18 (m, 1H, CH), 3.67 (d, 3 J HH =4.2Hz, 2H, OCH 2 ), 1.17(s, 24H, BOCMe 2 ), 1.09(d, 3 J HH = 6.0 Hz, 3H, Me). 13 C{ 1 H} NMR (151MHz, CDCl 3 ): δ82.70, 82.67 (BOCMe 2 ), 70.32 (OCH 2 ), 69.12(CH), 24.54, 24.53(BOCMe 2 ), 18.46 (Me). 11 B{ 1 H} NMR (193MHz, CDCl 3 ): δ22.19.
[0026] Available, the product structural formula is:
[0027]
Embodiment 3
[0029] The β-diimine monovalent magnesium compound catalyzes the reaction of 4-ethyl-1,3-dioxan-2-one with pinacol borane, and the process is as follows:
[0030] In the glove box, β-diimine monovalent magnesium compound 1mol%, 4-ethyl-1, 3-dioxan-2-one 0.4mmol, pinacol borane 1.6mmol, Then it was removed from the glove box, stirred for 6 h, and the yield was 99% according to nuclear magnetic spectrum.
[0031] Characterize the product, the data are: 1 H NMR (600MHz, CDCl 3 ): δ4.01-3.97 (m, 1H, CH), 3.75-3.65 (m, 2H, OCH2 ), 1.49-1.36 (m, 2H, CH 2 ), 1.17(s, 24H, BOCMe 2 ), 0.84(t, 3 J HH = 7.2Hz, 3H, Me). 13 C{ 1 H} NMR (151MHz, CDCl 3 ): δ82.58, 82.44 (BOCMe 2 ), 75.31(CH), 67.64(OCH 2 ), 25.21 (CH 2 ), 24.51, 24.48 (BOCMe 2 ), 9.40 (Me). 11 B{ 1 H} NMR (193MHz, CDCl 3 ): 622.24.
[0032] Available, the product structural formula is:
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com